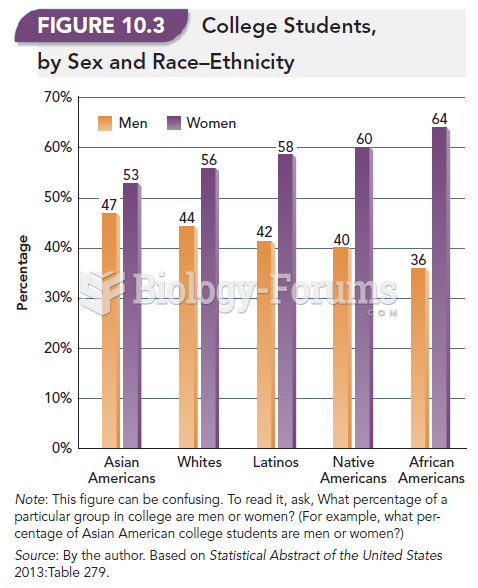
|
|
|
The average person is easily confused by the terms pharmaceutics and pharmacology, thinking they are one and the same. Whereas pharmaceutics is the science of preparing and dispensing drugs (otherwise known as the science of pharmacy), pharmacology is the study of medications.
The most destructive flu epidemic of all times in recorded history occurred in 1918, with approximately 20 million deaths worldwide.
The training of an anesthesiologist typically requires four years of college, 4 years of medical school, 1 year of internship, and 3 years of residency.
Oliver Wendell Holmes is credited with introducing the words "anesthesia" and "anesthetic" into the English language in 1846.
All patients with hyperparathyroidism will develop osteoporosis. The parathyroid glands maintain blood calcium within the normal range. All patients with this disease will continue to lose calcium from their bones every day, and there is no way to prevent the development of osteoporosis as a result.
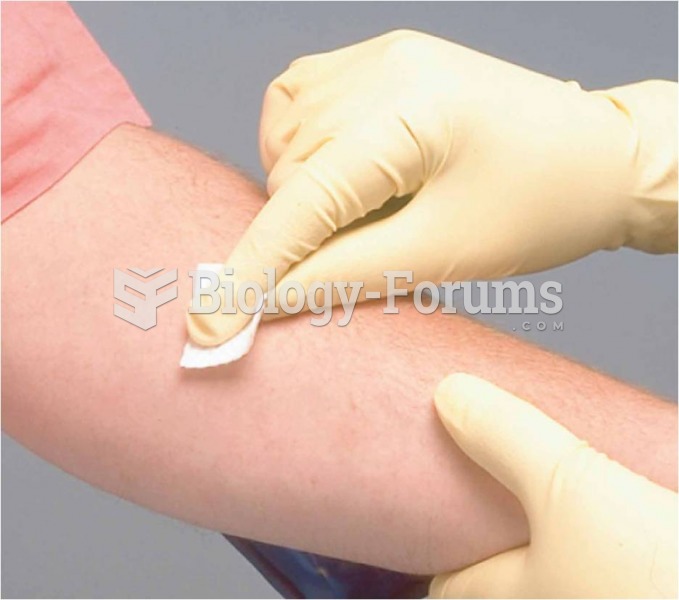 Intradermal drug administration: (b) the administration site is prepped Source: Pearson Education/PH
Intradermal drug administration: (b) the administration site is prepped Source: Pearson Education/PH
 In 1839 Mary Cragin, at the age of twenty-nine, became a convert to John Humphrey Noyes’s “communism
In 1839 Mary Cragin, at the age of twenty-nine, became a convert to John Humphrey Noyes’s “communism