|
|
|
When Gabriel Fahrenheit invented the first mercury thermometer, he called "zero degrees" the lowest temperature he was able to attain with a mixture of ice and salt. For the upper point of his scale, he used 96°, which he measured as normal human body temperature (we know it to be 98.6° today because of more accurate thermometers).
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.
When blood is exposed to air, it clots. Heparin allows the blood to come in direct contact with air without clotting.
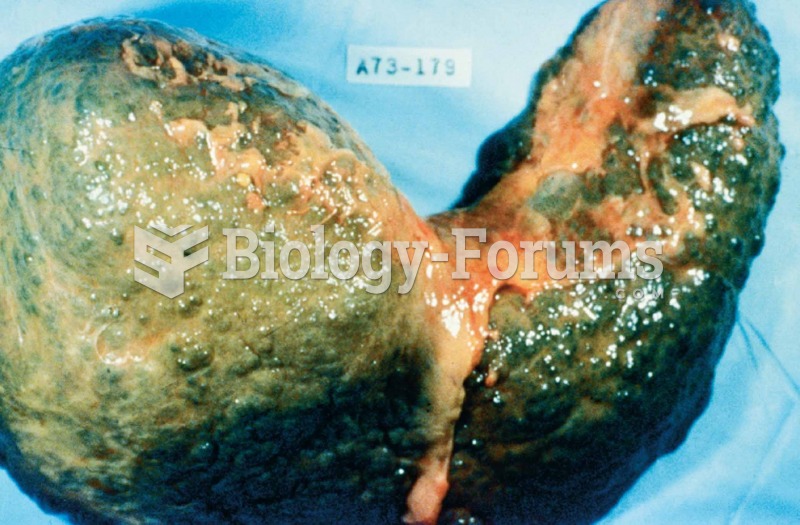
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.
It is widely believed that giving a daily oral dose of aspirin to heart attack patients improves their chances of survival because the aspirin blocks the formation of new blood clots.