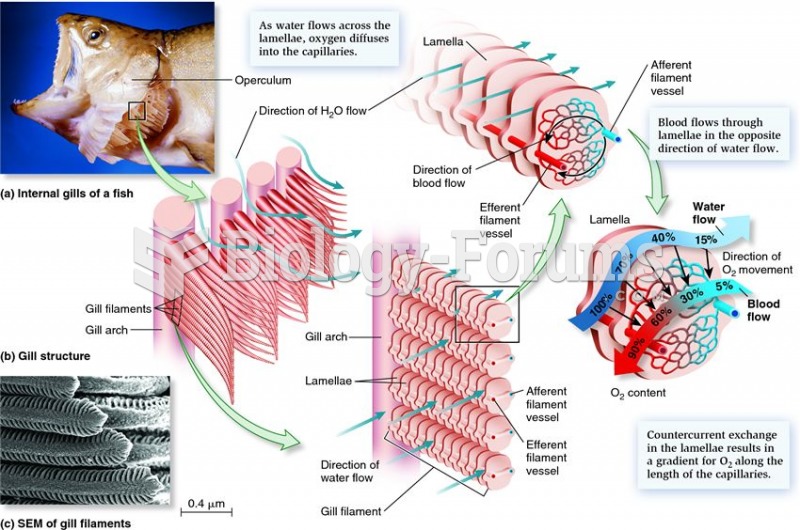
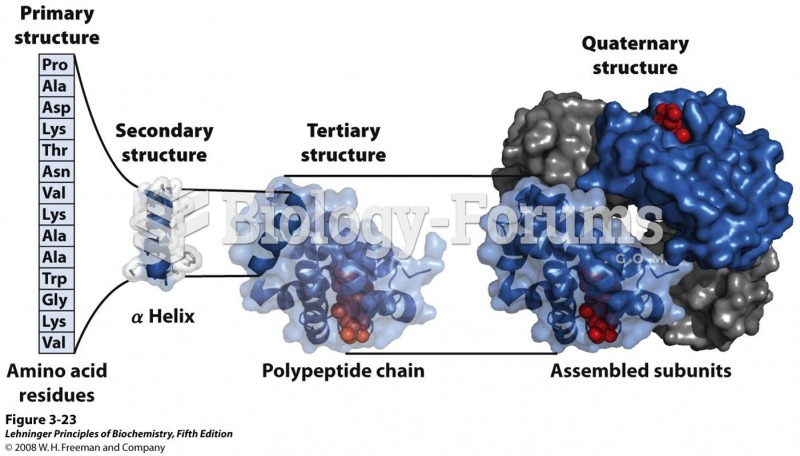
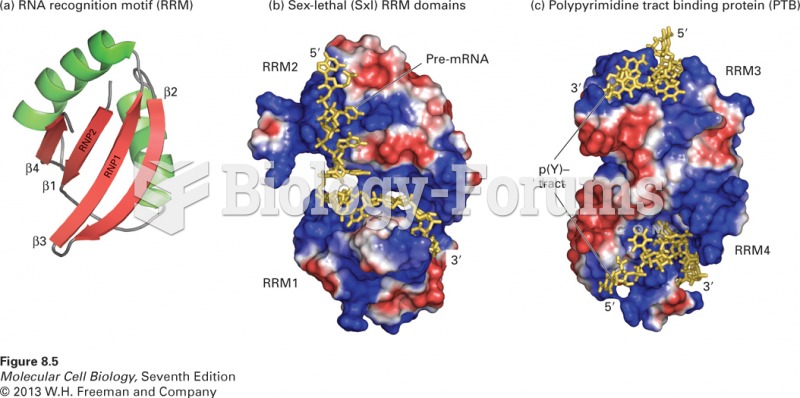
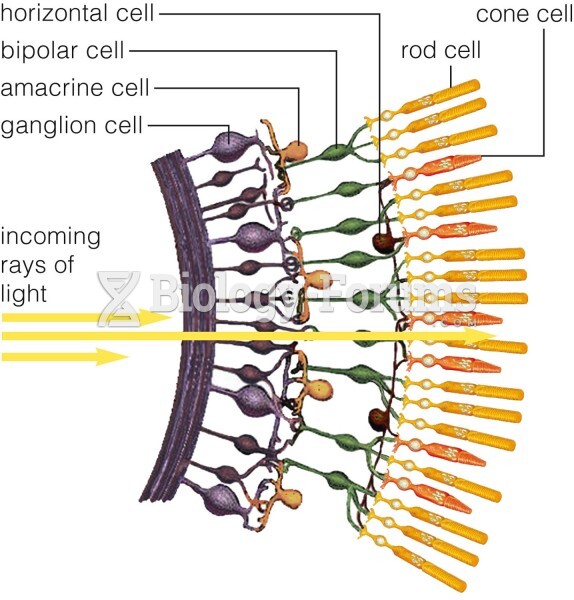
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.
Did you know?
Certain rare plants containing cyanide include apricot pits and a type of potato called cassava. Fortunately, only chronic or massive ingestion of any of these plants can lead to serious poisoning.
Did you know?
Approximately 25% of all reported medication errors result from some kind of name confusion.
Did you know?
Despite claims by manufacturers, the supplement known as Ginkgo biloba was shown in a study of more than 3,000 participants to be ineffective in reducing development of dementia and Alzheimer’s disease in older people.
Did you know?
It is believed that humans initially contracted crabs from gorillas about 3 million years ago from either sleeping in gorilla nests or eating the apes.