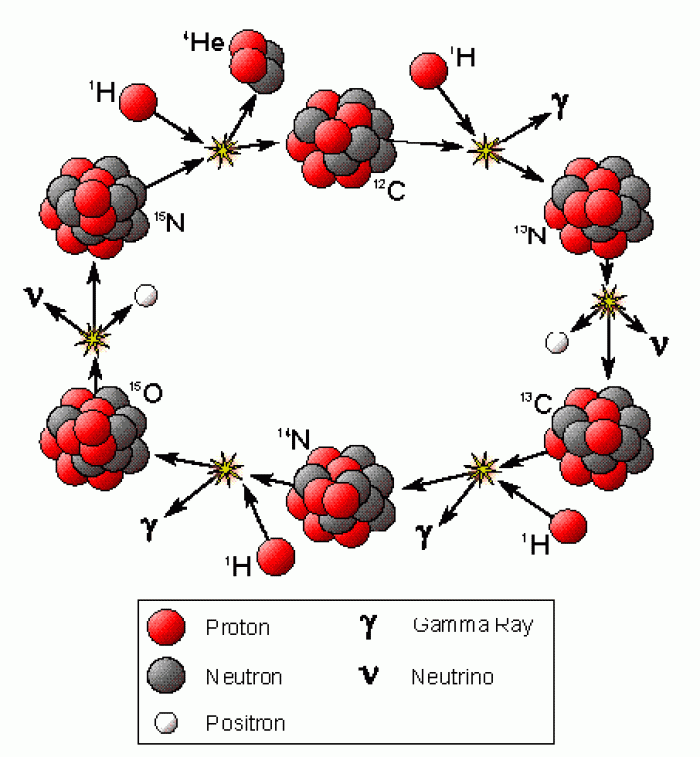
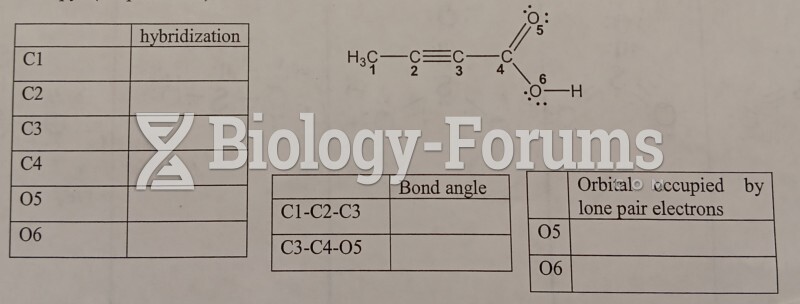
|
|
|
A headache when you wake up in the morning is indicative of sinusitis. Other symptoms of sinusitis can include fever, weakness, tiredness, a cough that may be more severe at night, and a runny nose or nasal congestion.
Approximately 500,000 babies are born each year in the United States to teenage mothers.
All patients with hyperparathyroidism will develop osteoporosis. The parathyroid glands maintain blood calcium within the normal range. All patients with this disease will continue to lose calcium from their bones every day, and there is no way to prevent the development of osteoporosis as a result.
During pregnancy, a woman is more likely to experience bleeding gums and nosebleeds caused by hormonal changes that increase blood flow to the mouth and nose.
In inpatient settings, adverse drug events account for an estimated one in three of all hospital adverse events. They affect approximately 2 million hospital stays every year, and prolong hospital stays by between one and five days.
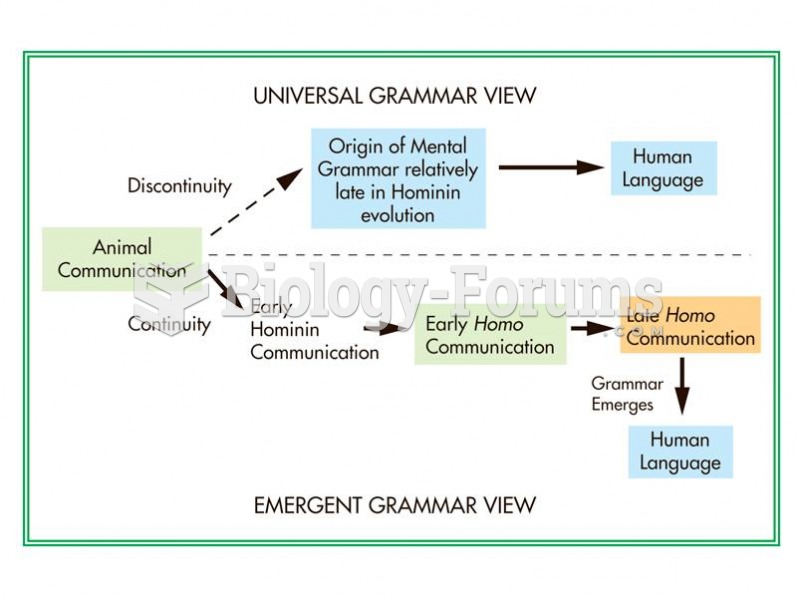 The universal grammar and emergent grammar viewpoint lead to very different scenarios of the evoluti
The universal grammar and emergent grammar viewpoint lead to very different scenarios of the evoluti
 Some playgrounds near streets with heavy traffic may still have high levels of lead from gasoline ...
Some playgrounds near streets with heavy traffic may still have high levels of lead from gasoline ...