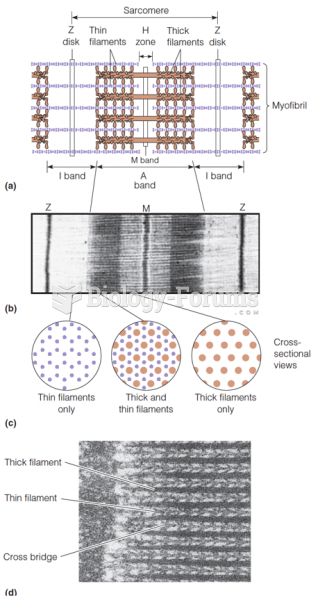
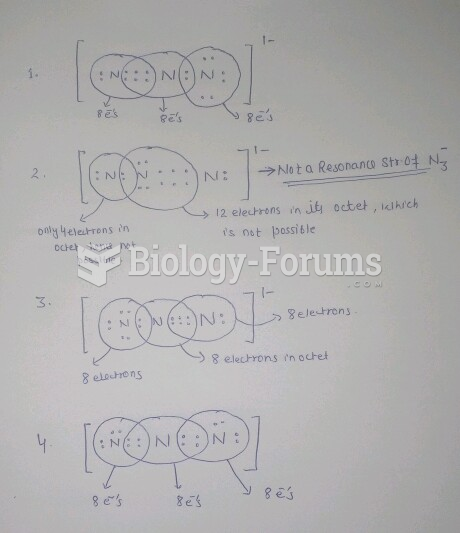
|
|
|
Opium has influenced much of the world's most popular literature. The following authors were all opium users, of varying degrees: Lewis Carroll, Charles, Dickens, Arthur Conan Doyle, and Oscar Wilde.
There are actually 60 minerals, 16 vitamins, 12 essential amino acids, and three essential fatty acids that your body needs every day.
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.
Immunoglobulin injections may give short-term protection against, or reduce severity of certain diseases. They help people who have an inherited problem making their own antibodies, or those who are having certain types of cancer treatments.