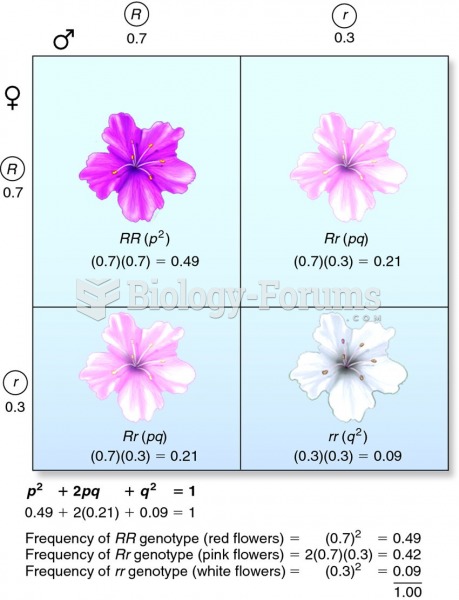
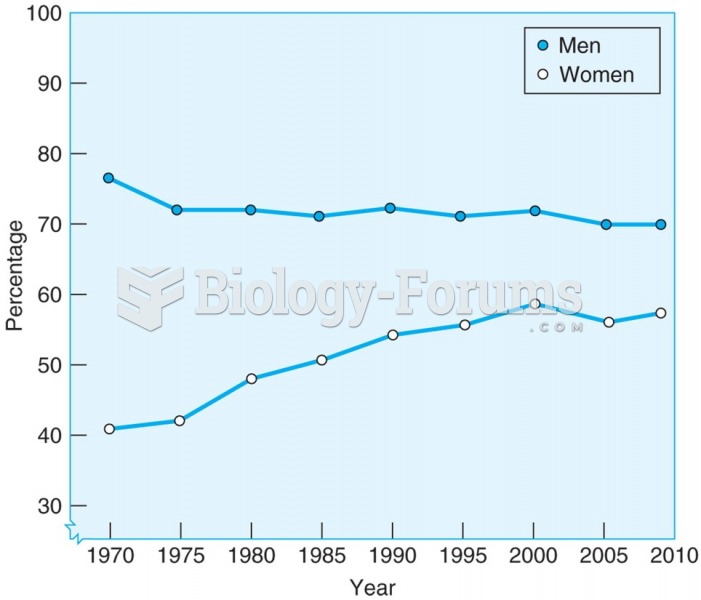
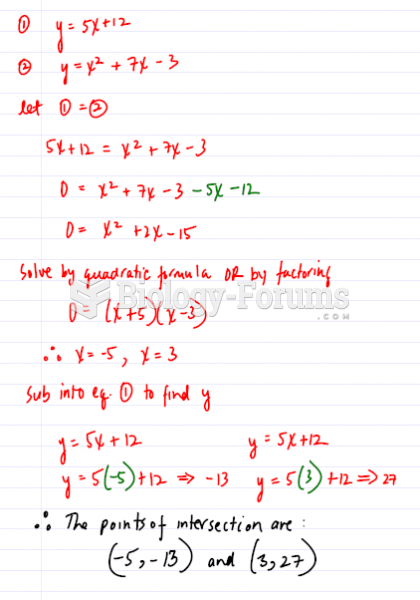|
|
|
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.
Patients should never assume they are being given the appropriate drugs. They should make sure they know which drugs are being prescribed, and always double-check that the drugs received match the prescription.
The highest suicide rate in the United States is among people ages 65 years and older. Almost 15% of people in this age group commit suicide every year.
In inpatient settings, adverse drug events account for an estimated one in three of all hospital adverse events. They affect approximately 2 million hospital stays every year, and prolong hospital stays by between one and five days.
Each year in the United States, there are approximately six million pregnancies. This means that at any one time, about 4% of women in the United States are pregnant.