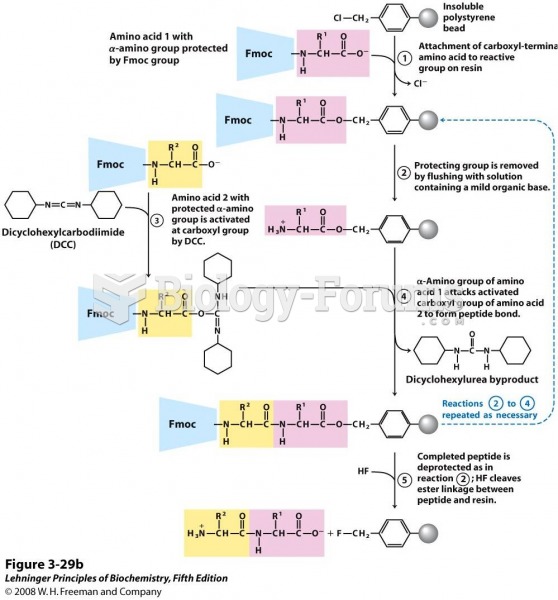
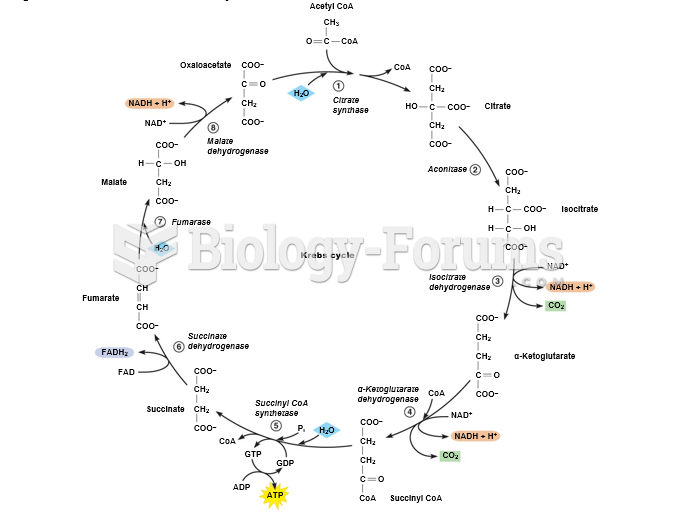
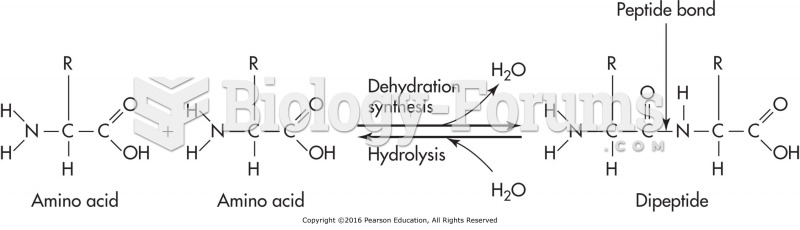
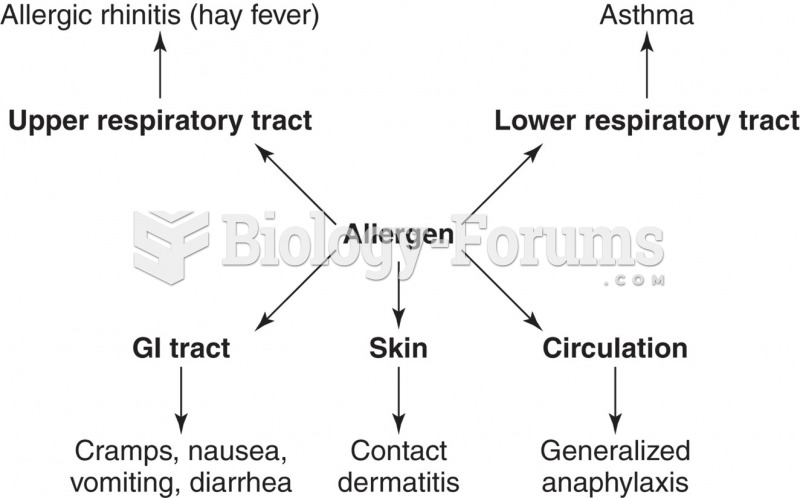
|
|
|
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
Walt Disney helped combat malaria by making an animated film in 1943 called The Winged Scourge. This short film starred the seven dwarfs and taught children that mosquitos transmit malaria, which is a very bad disease. It advocated the killing of mosquitos to stop the disease.
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
Patients who have undergone chemotherapy for the treatment of cancer often complain of a lack of mental focus; memory loss; and a general diminution in abilities such as multitasking, attention span, and general mental agility.
In women, pharmacodynamic differences include increased sensitivity to (and increased effectiveness of) beta-blockers, opioids, selective serotonin reuptake inhibitors, and typical antipsychotics.