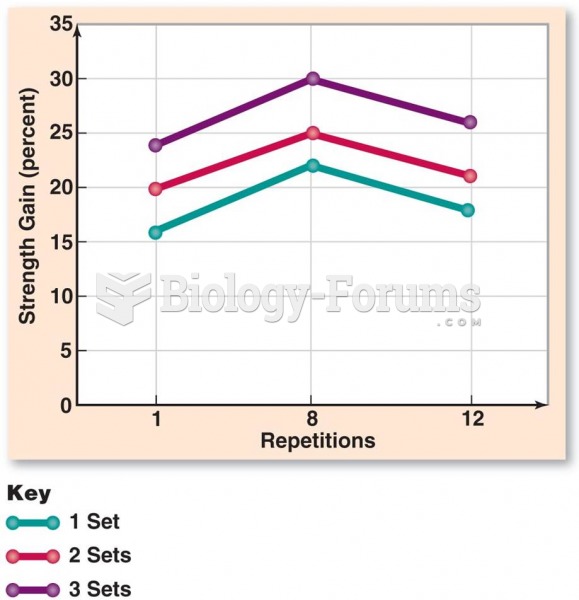
|
|
|
Studies show that systolic blood pressure can be significantly lowered by taking statins. In fact, the higher the patient's baseline blood pressure, the greater the effect of statins on his or her blood pressure.
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
According to the CDC, approximately 31.7% of the U.S. population has high low-density lipoprotein (LDL) or "bad cholesterol" levels.
Drug-induced pharmacodynamic effects manifested in older adults include drug-induced renal toxicity, which can be a major factor when these adults are experiencing other kidney problems.
The effects of organophosphate poisoning are referred to by using the abbreviations “SLUD” or “SLUDGE,” It stands for: salivation, lacrimation, urination, defecation, GI upset, and emesis.
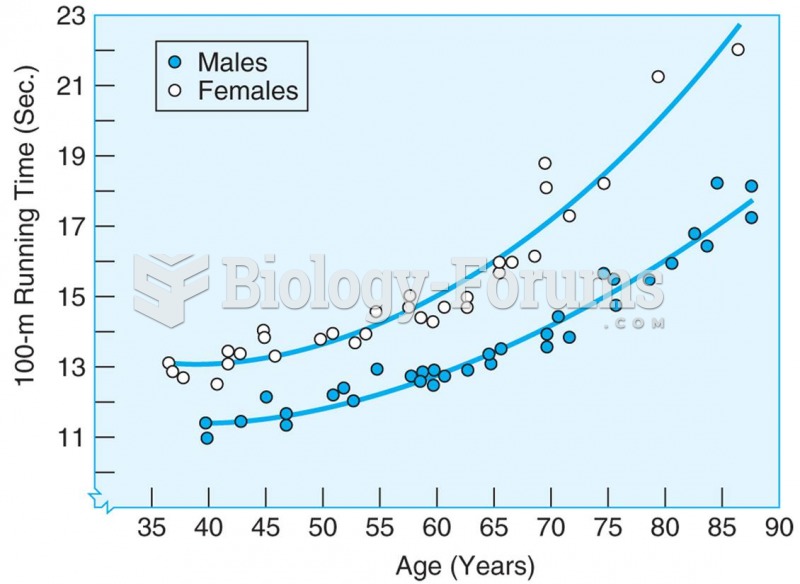 Running time on 100-meter sprint for men and women master athletes increases with age. Source: Korho
Running time on 100-meter sprint for men and women master athletes increases with age. Source: Korho
 The 2012 U.S. presidential campaign rang with “get tough” cries to end Iran’s nuclear capacity and c
The 2012 U.S. presidential campaign rang with “get tough” cries to end Iran’s nuclear capacity and c