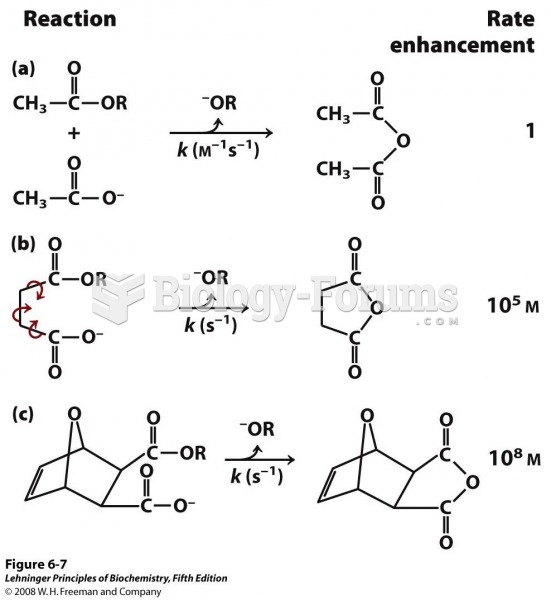
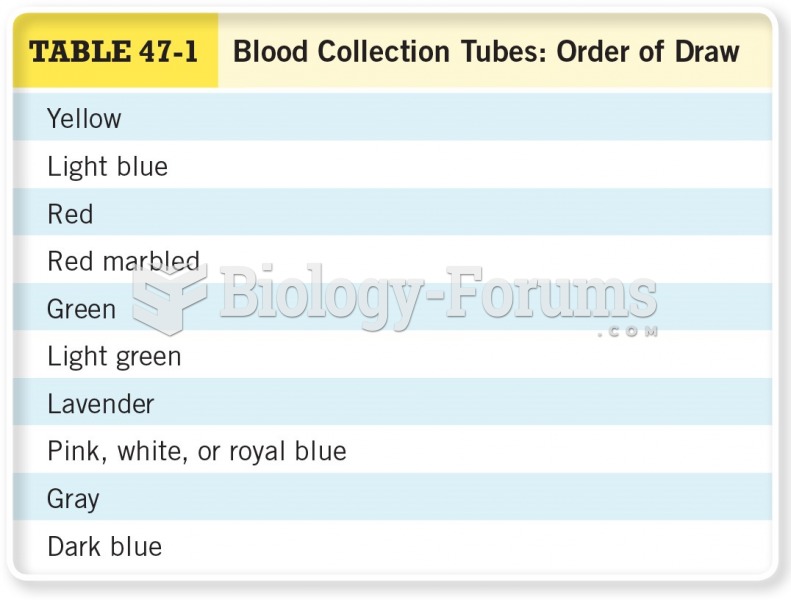
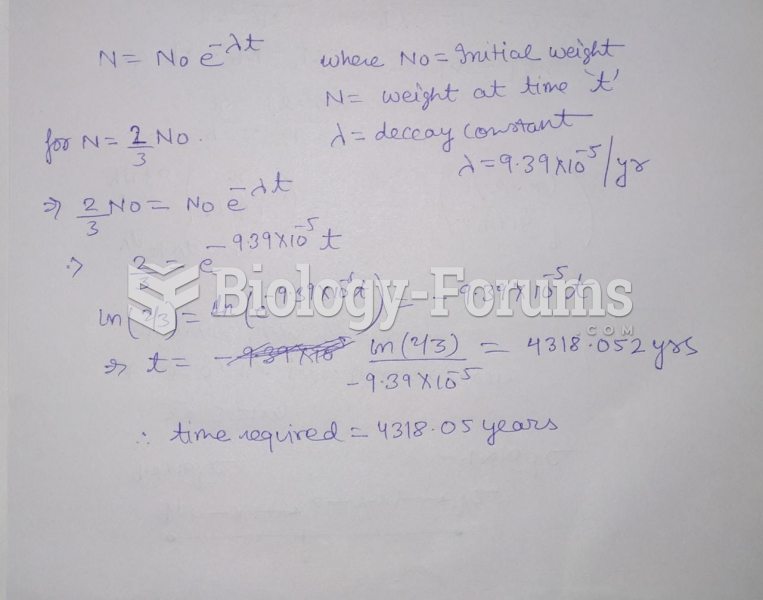
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.
Did you know?
Studies show that systolic blood pressure can be significantly lowered by taking statins. In fact, the higher the patient's baseline blood pressure, the greater the effect of statins on his or her blood pressure.
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
According to the Migraine Research Foundation, migraines are the third most prevalent illness in the world. Women are most affected (18%), followed by children of both sexes (10%), and men (6%).
Did you know?
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.