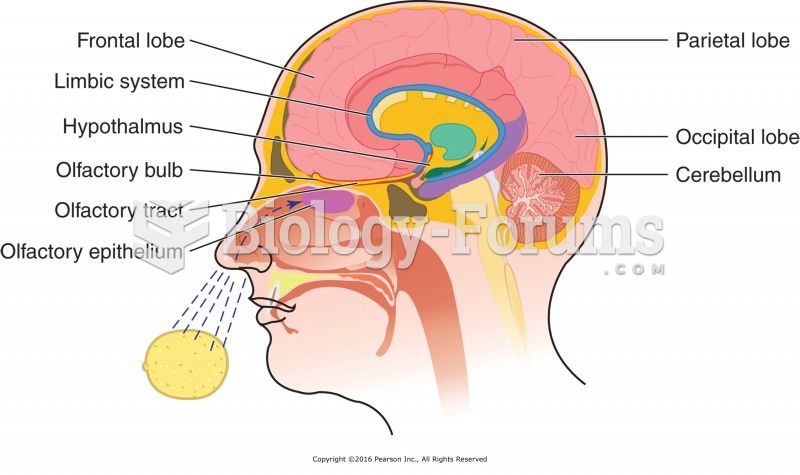
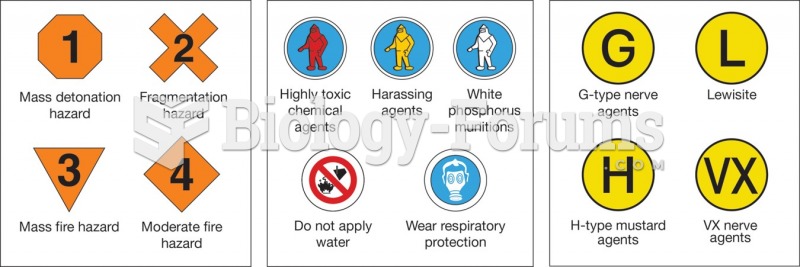
|
|
|
An identified risk factor for osteoporosis is the intake of excessive amounts of vitamin A. Dietary intake of approximately double the recommended daily amount of vitamin A, by women, has been shown to reduce bone mineral density and increase the chances for hip fractures compared with women who consumed the recommended daily amount (or less) of vitamin A.
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
Glaucoma is a leading cause of blindness. As of yet, there is no cure. Everyone is at risk, and there may be no warning signs. It is six to eight times more common in African Americans than in whites. The best and most effective way to detect glaucoma is to receive a dilated eye examination.
Women are 50% to 75% more likely than men to experience an adverse drug reaction.