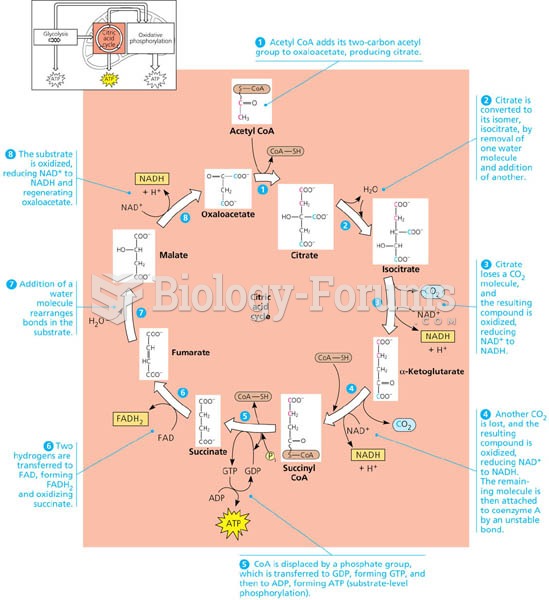
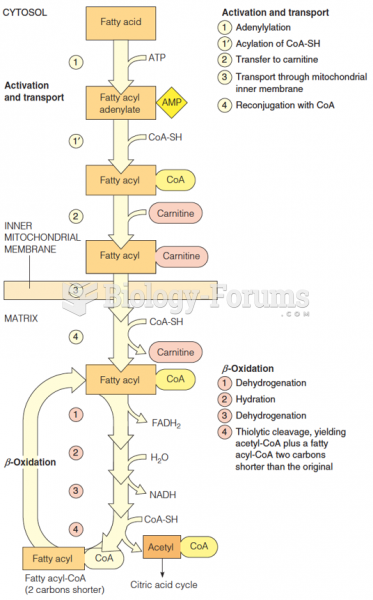
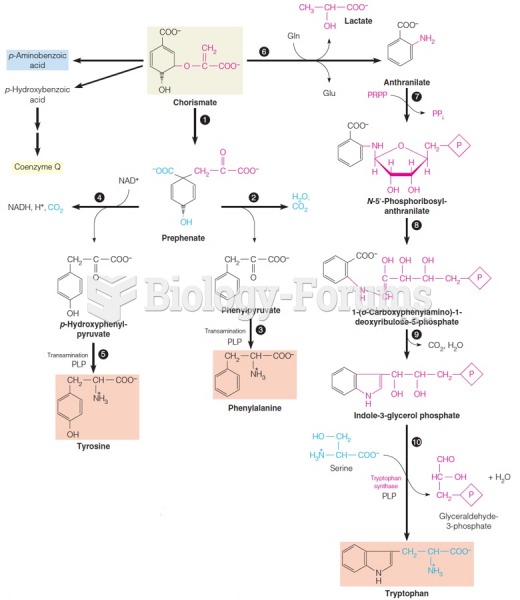
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Alcohol acts as a diuretic. Eight ounces of water is needed to metabolize just 1 ounce of alcohol.
Did you know?
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
Did you know?
Always store hazardous household chemicals in their original containers out of reach of children. These include bleach, paint, strippers and products containing turpentine, garden chemicals, oven cleaners, fondue fuels, nail polish, and nail polish remover.
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.
Did you know?
Human kidneys will clean about 1 million gallons of blood in an average lifetime.