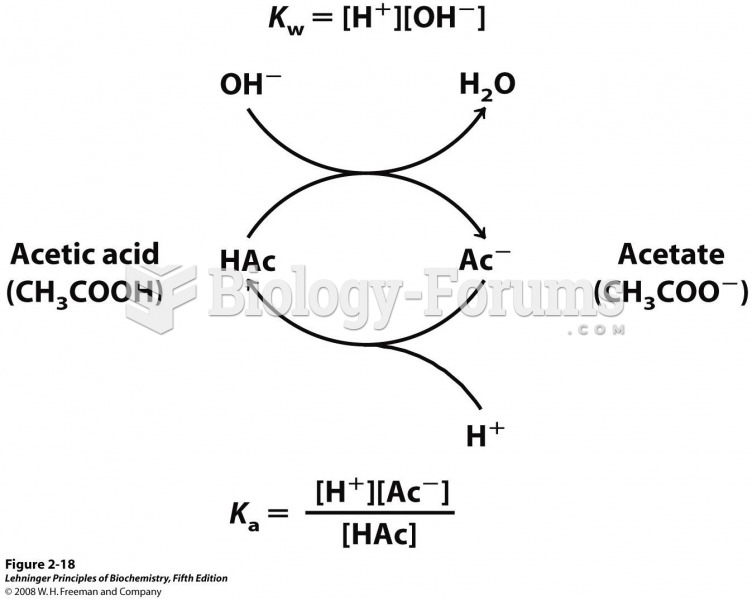
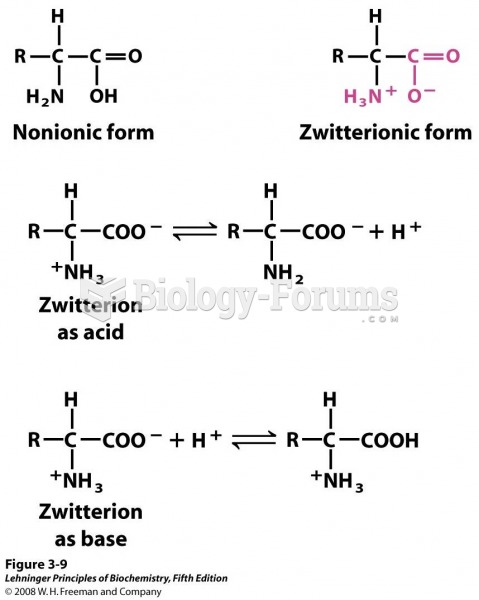
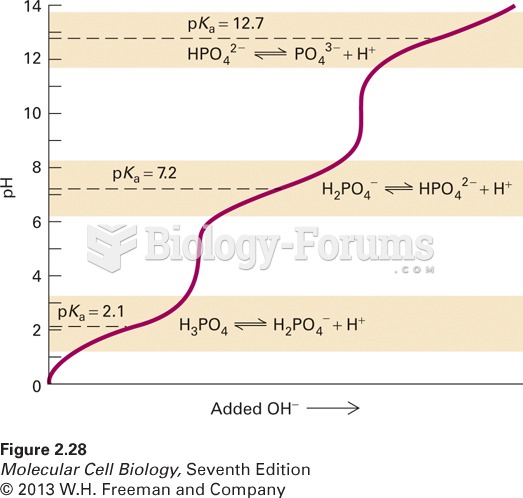
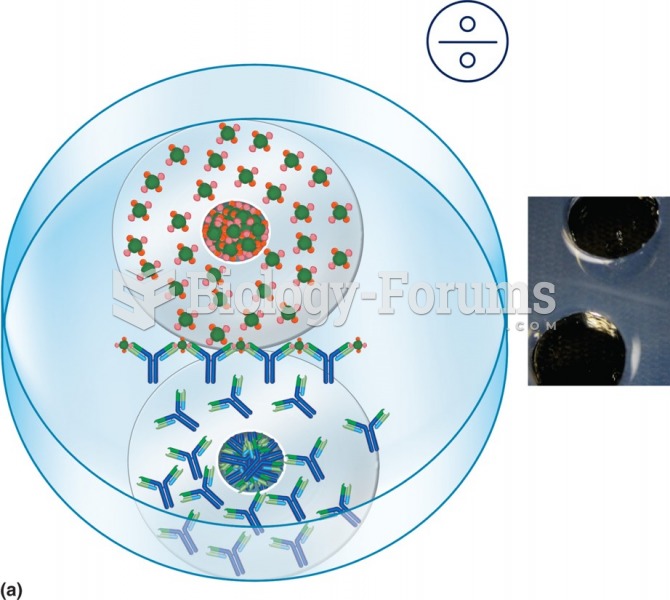
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In 1885, the Lloyd Manufacturing Company of Albany, New York, promoted and sold "Cocaine Toothache Drops" at 15 cents per bottle! In 1914, the Harrison Narcotic Act brought the sale and distribution of this drug under federal control.
Did you know?
Normal urine is sterile. It contains fluids, salts, and waste products. It is free of bacteria, viruses, and fungi.
Did you know?
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
Did you know?
The lipid bilayer is made of phospholipids. They are arranged in a double layer because one of their ends is attracted to water while the other is repelled by water.
Did you know?
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.