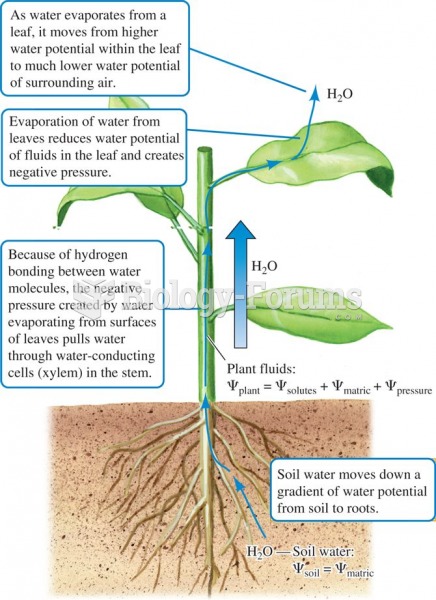
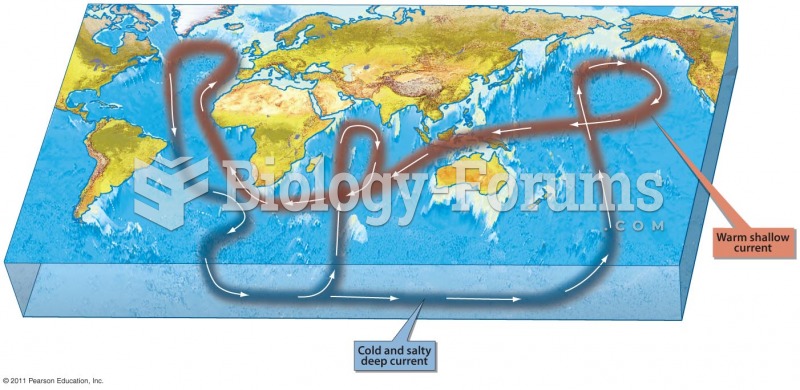
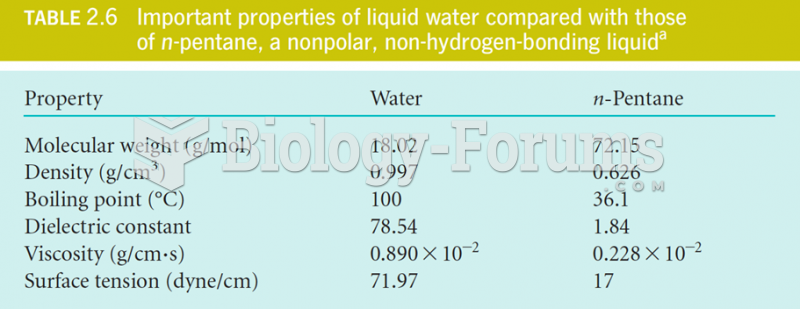
|
|
|
Elderly adults are at greatest risk of stroke and myocardial infarction and have the most to gain from prophylaxis. Patients ages 60 to 80 years with blood pressures above 160/90 mm Hg should benefit from antihypertensive treatment.
Signs and symptoms that may signify an eye tumor include general blurred vision, bulging eye(s), double vision, a sensation of a foreign body in the eye(s), iris defects, limited ability to move the eyelid(s), limited ability to move the eye(s), pain or discomfort in or around the eyes or eyelids, red or pink eyes, white or cloud spots on the eye(s), colored spots on the eyelid(s), swelling around the eyes, swollen eyelid(s), and general vision loss.
When taking monoamine oxidase inhibitors, people should avoid a variety of foods, which include alcoholic beverages, bean curd, broad (fava) bean pods, cheese, fish, ginseng, protein extracts, meat, sauerkraut, shrimp paste, soups, and yeast.
Parkinson's disease is both chronic and progressive. This means that it persists over a long period of time and that its symptoms grow worse over time.
Asthma is the most common chronic childhood disease in the world. Most children who develop asthma have symptoms before they are 5 years old.