|
|
|
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.
More than 20 million Americans cite use of marijuana within the past 30 days, according to the National Survey on Drug Use and Health (NSDUH). More than 8 million admit to using it almost every day.
Vampire bats have a natural anticoagulant in their saliva that permits continuous bleeding after they painlessly open a wound with their incisors. This capillary blood does not cause any significant blood loss to their victims.
Approximately 15–25% of recognized pregnancies end in miscarriage. However, many miscarriages often occur before a woman even knows she is pregnant.
Always store hazardous household chemicals in their original containers out of reach of children. These include bleach, paint, strippers and products containing turpentine, garden chemicals, oven cleaners, fondue fuels, nail polish, and nail polish remover.
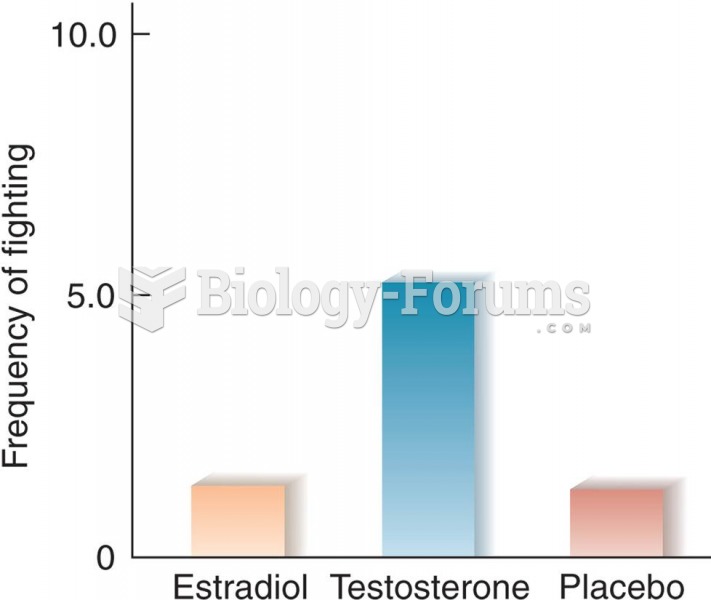 Effects of Estradiol and Testosterone on Interfemale Aggression in Rats (Based on data from van de P
Effects of Estradiol and Testosterone on Interfemale Aggression in Rats (Based on data from van de P
 The material safety data sheet (MSDS) for sulfuric acid showing the detailed technical information ...
The material safety data sheet (MSDS) for sulfuric acid showing the detailed technical information ...