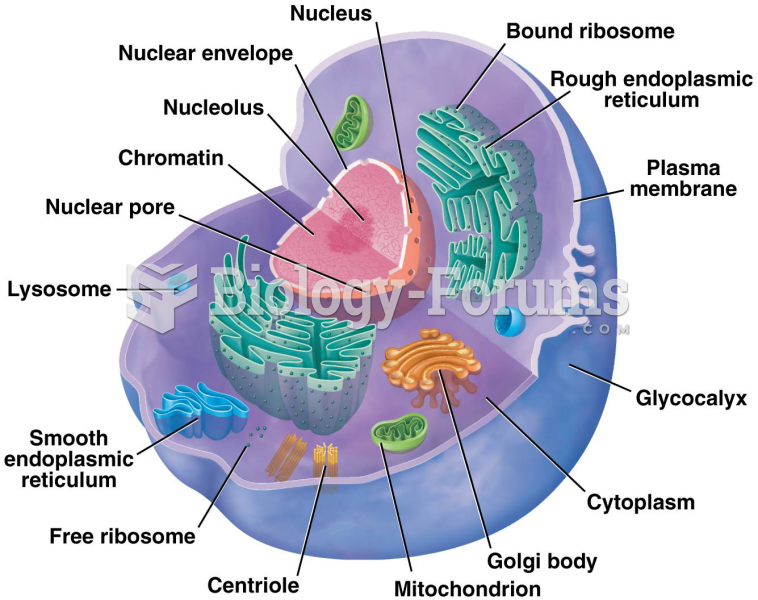
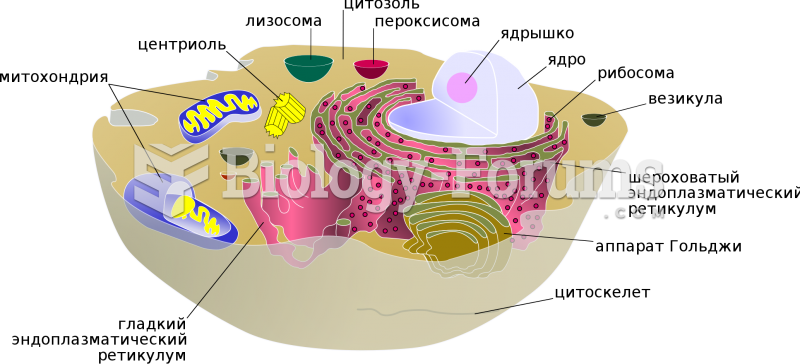
|
|
|
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
Bacteria have been found alive in a lake buried one half mile under ice in Antarctica.
Every 10 seconds, a person in the United States goes to the emergency room complaining of head pain. About 1.2 million visits are for acute migraine attacks.
Chronic necrotizing aspergillosis has a slowly progressive process that, unlike invasive aspergillosis, does not spread to other organ systems or the blood vessels. It most often affects middle-aged and elderly individuals, spreading to surrounding tissue in the lungs. The disease often does not respond to conventionally successful treatments, and requires individualized therapies in order to keep it from becoming life-threatening.
There are approximately 3 million unintended pregnancies in the United States each year.