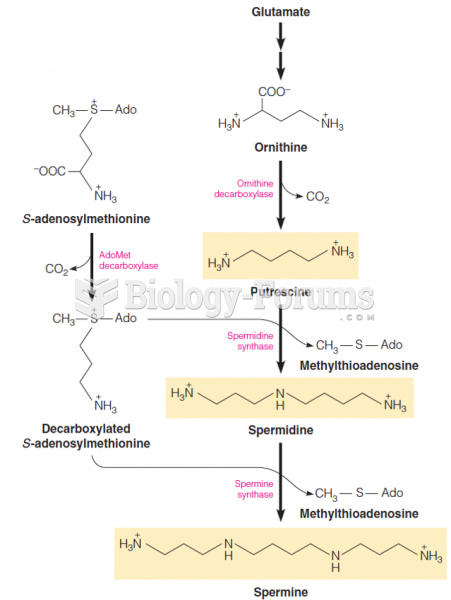
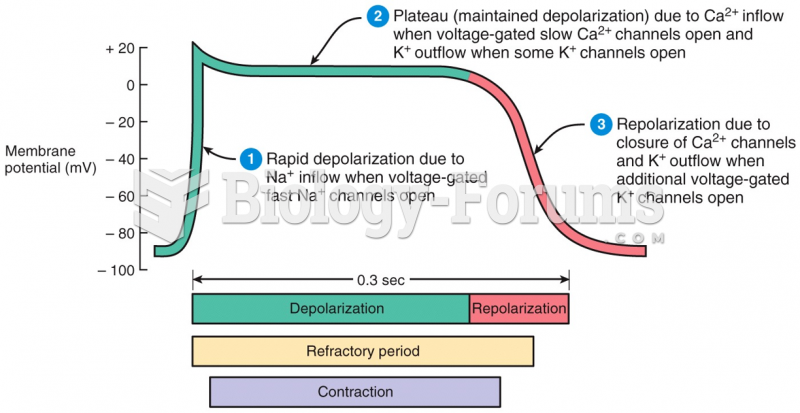
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Approximately 25% of all reported medication errors result from some kind of name confusion.
Did you know?
People about to have surgery must tell their health care providers about all supplements they take.
Did you know?
According to the National Institute of Environmental Health Sciences, lung disease is the third leading killer in the United States, responsible for one in seven deaths. It is the leading cause of death among infants under the age of one year.
Did you know?
Colchicine is a highly poisonous alkaloid originally extracted from a type of saffron plant that is used mainly to treat gout.
Did you know?
Alcohol acts as a diuretic. Eight ounces of water is needed to metabolize just 1 ounce of alcohol.