|
|
|
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
A seasonal flu vaccine is the best way to reduce the chances you will get seasonal influenza and spread it to others.
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.
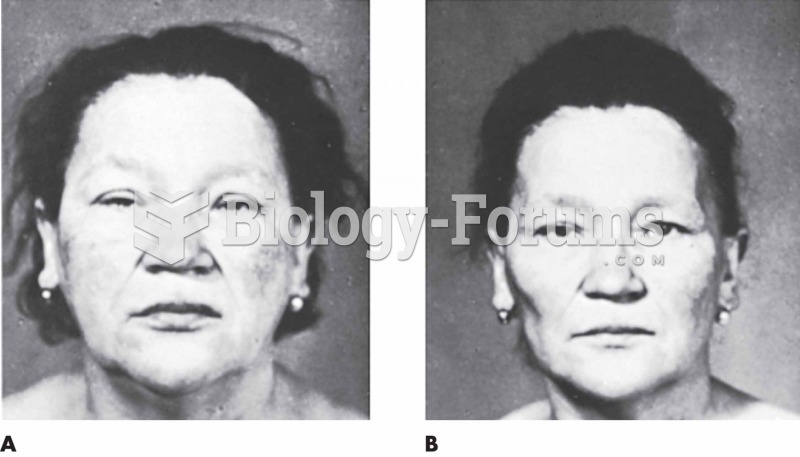
The toxic levels for lithium carbonate are close to the therapeutic levels. Signs of toxicity include fine hand tremor, polyuria, mild thirst, nausea, general discomfort, diarrhea, vomiting, drowsiness, muscular weakness, lack of coordination, ataxia, giddiness, tinnitus, and blurred vision.