|
|
|
People with alcoholism are at a much greater risk of malnutrition than are other people and usually exhibit low levels of most vitamins (especially folic acid). This is because alcohol often takes the place of 50% of their daily intake of calories, with little nutritional value contained in it.
Side effects from substance abuse include nausea, dehydration, reduced productivitiy, and dependence. Though these effects usually worsen over time, the constant need for the substance often overcomes rational thinking.
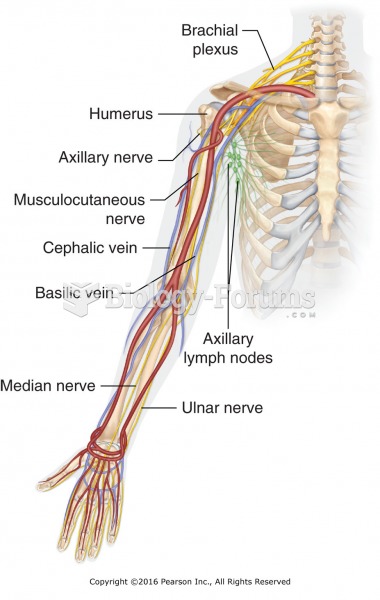
The heart is located in the center of the chest, with part of it tipped slightly so that it taps against the left side of the chest.
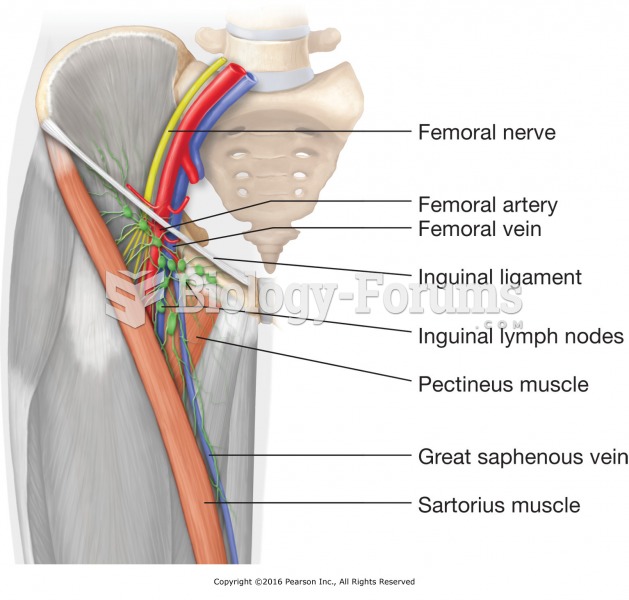
Persons who overdose with cardiac glycosides have a better chance of overall survival if they can survive the first 24 hours after the overdose.
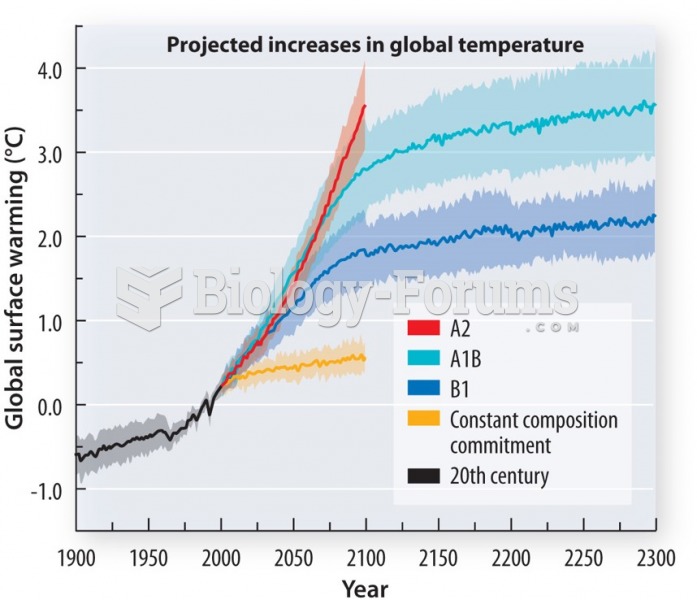
During the twentieth century, a variant of the metric system was used in Russia and France in which the base unit of mass was the tonne. Instead of kilograms, this system used millitonnes (mt).
 Pressure testing the cooling system. A typical hand-operated pressure tester applies pressure equal ...
Pressure testing the cooling system. A typical hand-operated pressure tester applies pressure equal ...
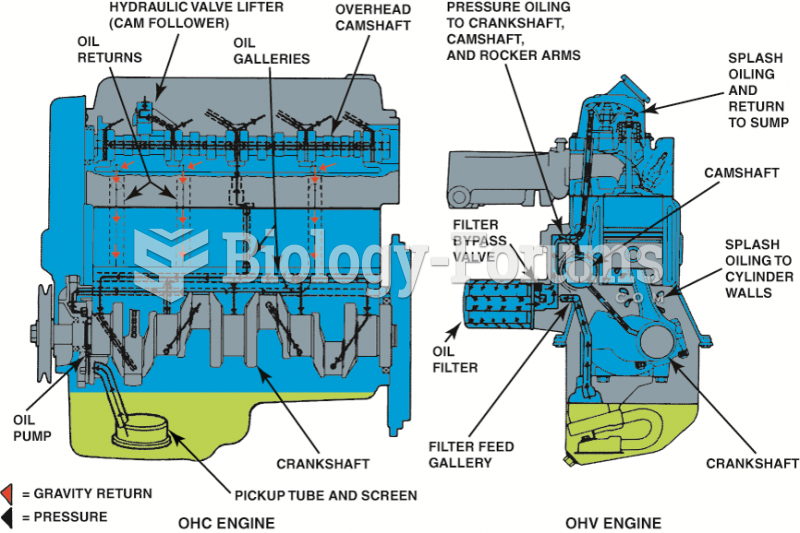 A typical engine design that uses both pressure and splash lubrication. Oil travels under pressure ...
A typical engine design that uses both pressure and splash lubrication. Oil travels under pressure ...