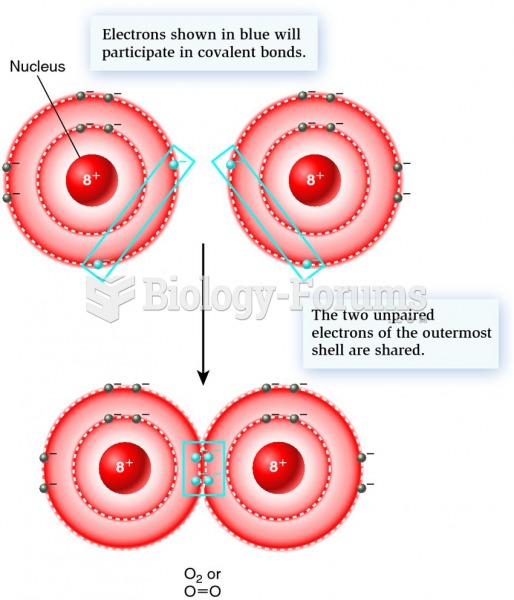
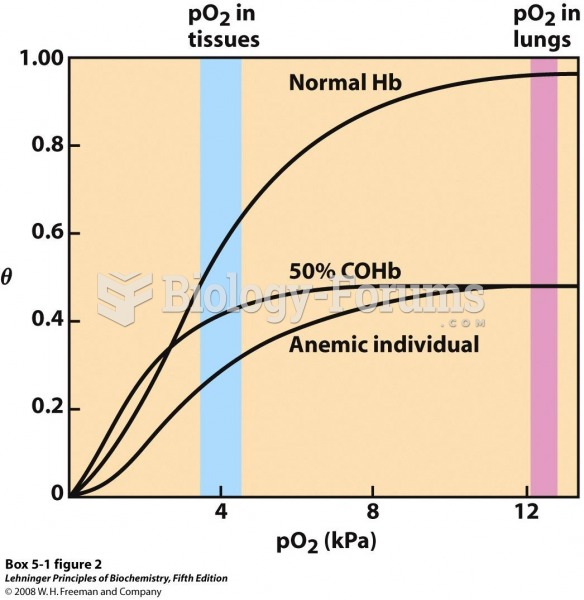
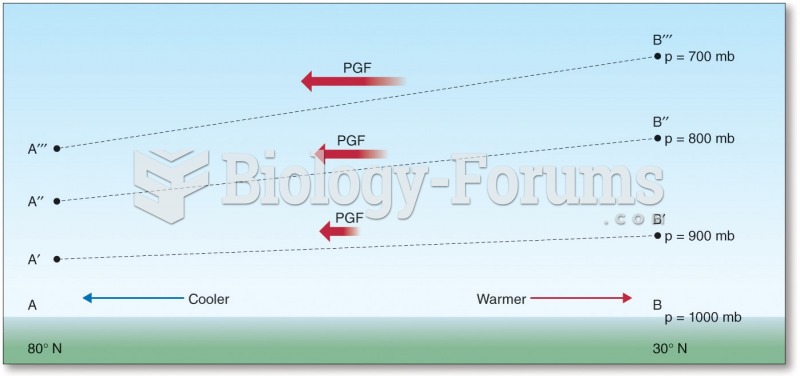
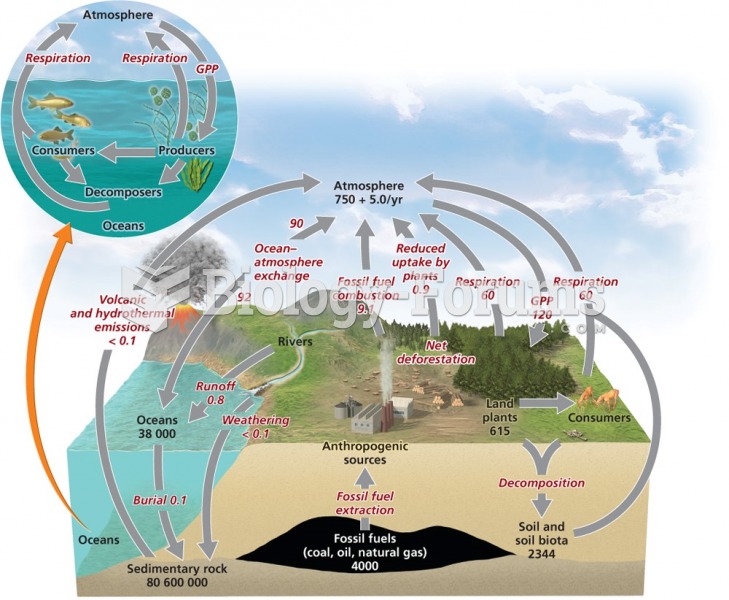
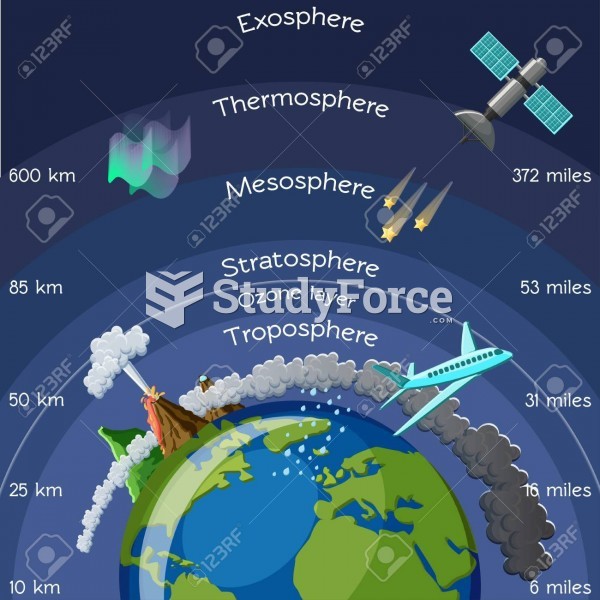
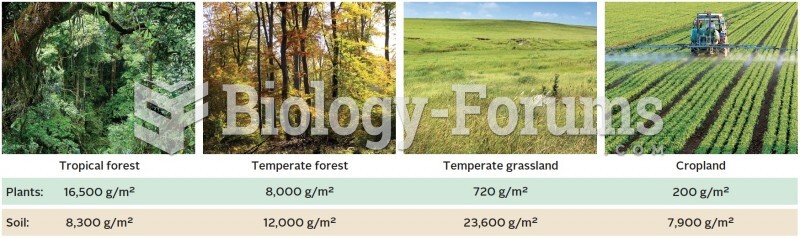
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The average adult has about 21 square feet of skin.
Did you know?
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.
Did you know?
Essential fatty acids have been shown to be effective against ulcers, asthma, dental cavities, and skin disorders such as acne.
Did you know?
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
Did you know?
The effects of organophosphate poisoning are referred to by using the abbreviations “SLUD” or “SLUDGE,” It stands for: salivation, lacrimation, urination, defecation, GI upset, and emesis.