|
|
|
Illicit drug use costs the United States approximately $181 billion every year.
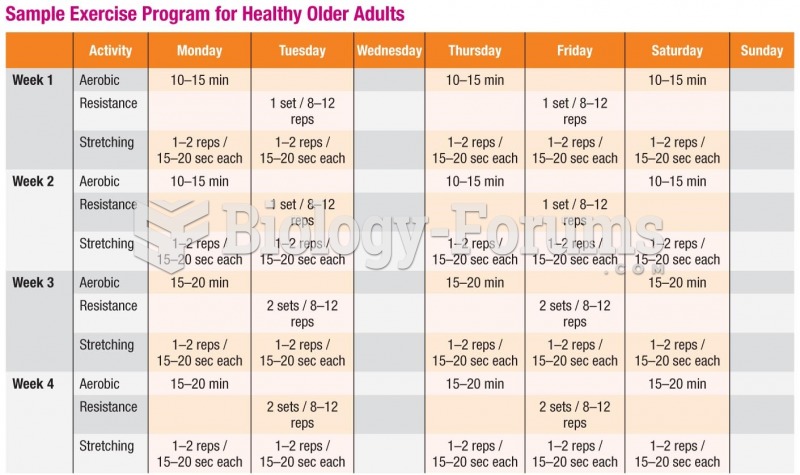
Vital signs (blood pressure, temperature, pulse rate, respiration rate) should be taken before any drug administration. Patients should be informed not to use tobacco or caffeine at least 30 minutes before their appointment.
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
The human body's pharmacokinetics are quite varied. Our hair holds onto drugs longer than our urine, blood, or saliva. For example, alcohol can be detected in the hair for up to 90 days after it was consumed. The same is true for marijuana, cocaine, ecstasy, heroin, methamphetamine, and nicotine.