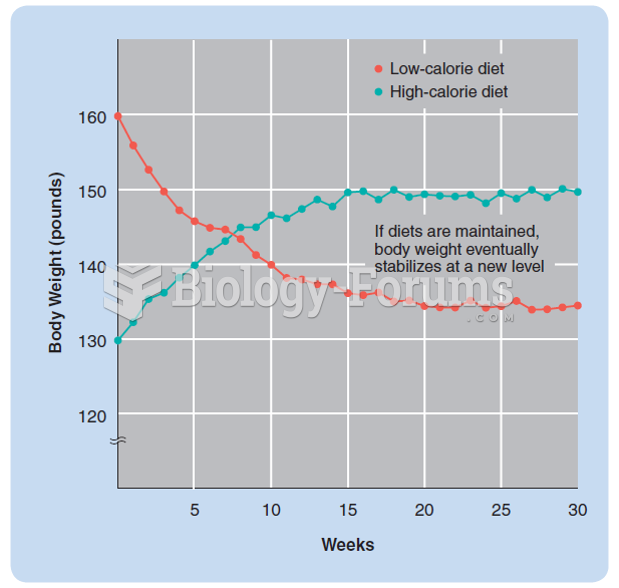
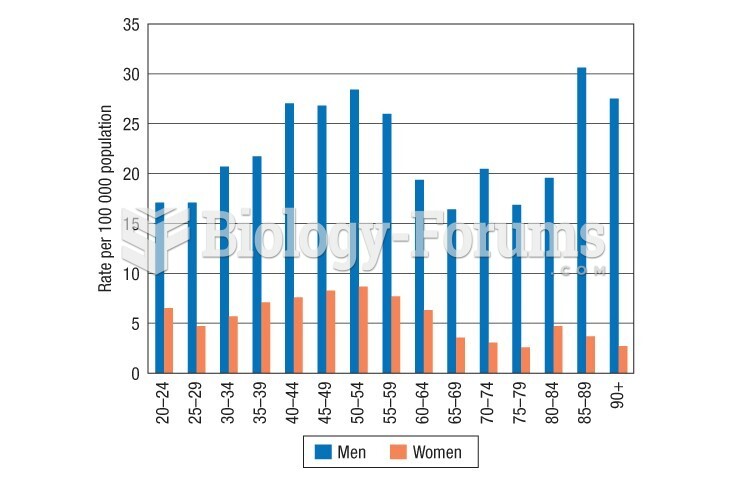
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Oliver Wendell Holmes is credited with introducing the words "anesthesia" and "anesthetic" into the English language in 1846.
Did you know?
Symptoms of kidney problems include a loss of appetite, back pain (which may be sudden and intense), chills, abdominal pain, fluid retention, nausea, the urge to urinate, vomiting, and fever.
Did you know?
Human kidneys will clean about 1 million gallons of blood in an average lifetime.
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.
Did you know?
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.