|
|
|
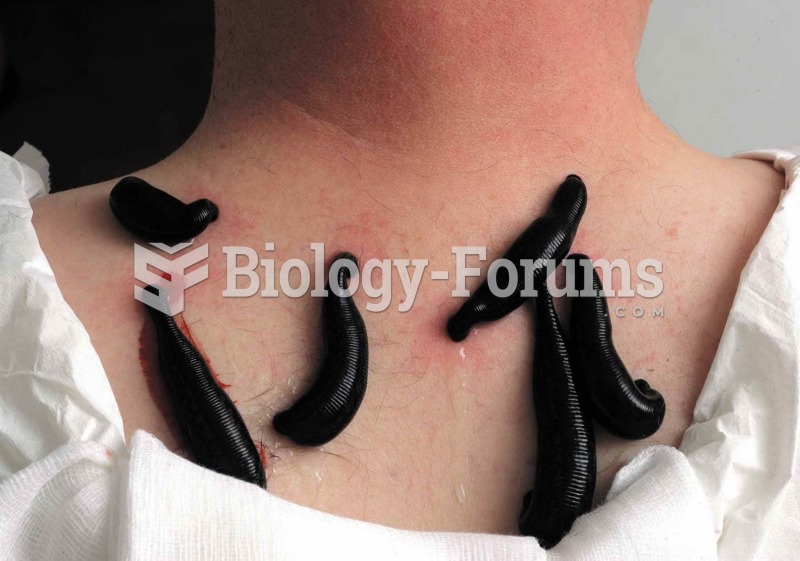
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
Of the estimated 2 million heroin users in the United States, 600,000–800,000 are considered hardcore addicts. Heroin addiction is considered to be one of the hardest addictions to recover from.
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
Tobacco depletes the body of vitamins A, C, and E, which can result in any of the following: dry hair, dry skin, dry eyes, poor growth, night blindness, abscesses, insomnia, fatigue, reproductive system problems, sinusitis, pneumonia, frequent respiratory problems, skin disorders, weight loss, rickets, osteomalacia, nervousness, muscle spasms, leg cramps, extremity numbness, bone malformations, decayed teeth, difficulty in walking, irritability, restlessness, profuse sweating, increased uric acid (gout), joint damage, damaged red blood cells, destruction of nerves, infertility, miscarriage, and many types of cancer.
IgA antibodies protect body surfaces exposed to outside foreign substances. IgG antibodies are found in all body fluids. IgM antibodies are the first type of antibody made in response to an infection. IgE antibody levels are often high in people with allergies. IgD antibodies are found in tissues lining the abdomen and chest.
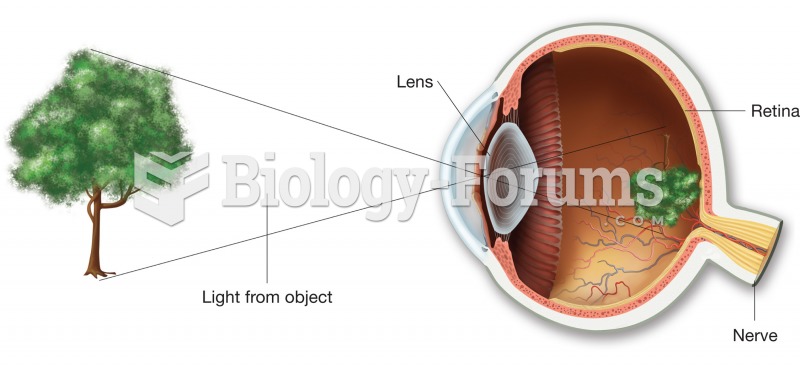 The image formed on the retina is inverted. The brain rights the image as part of the interpretation
The image formed on the retina is inverted. The brain rights the image as part of the interpretation
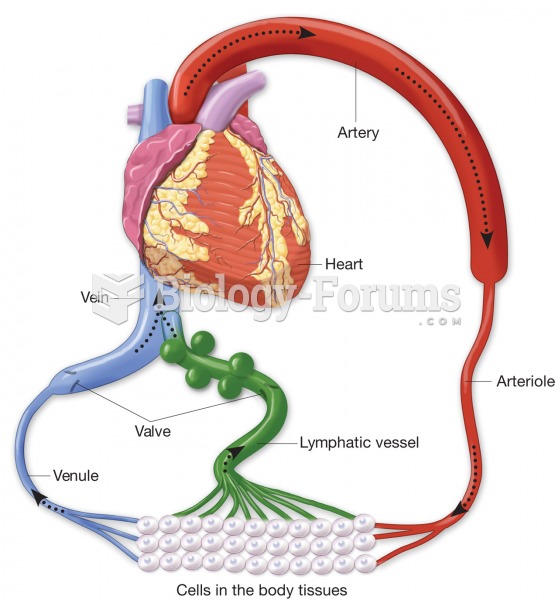 Lymphatic vessels (green) pick up excess tissue fluid, purify it in lymph nodes, and return it to th
Lymphatic vessels (green) pick up excess tissue fluid, purify it in lymph nodes, and return it to th