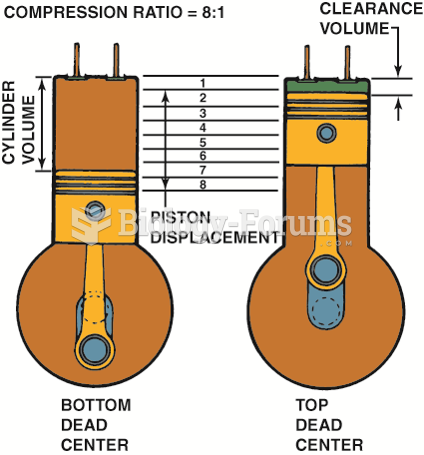
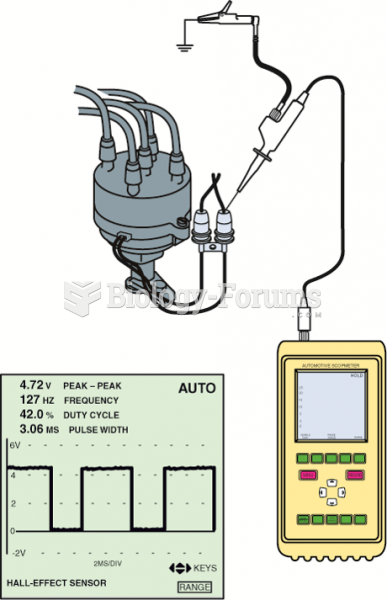
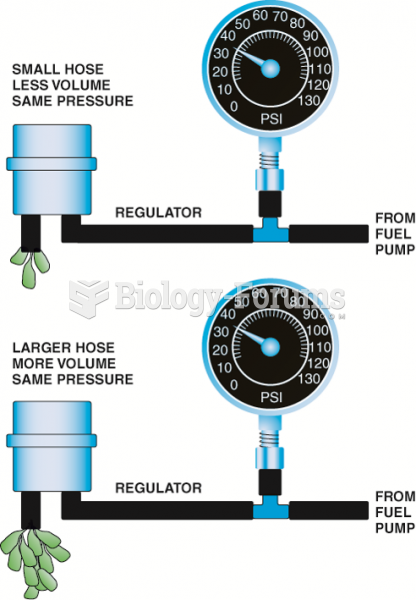
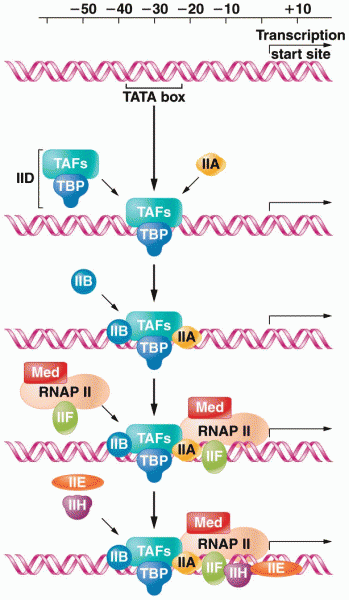
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Throughout history, plants containing cardiac steroids have been used as heart drugs and as poisons (e.g., in arrows used in combat), emetics, and diuretics.
Did you know?
People about to have surgery must tell their health care providers about all supplements they take.
Did you know?
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.
Did you know?
Immunoglobulin injections may give short-term protection against, or reduce severity of certain diseases. They help people who have an inherited problem making their own antibodies, or those who are having certain types of cancer treatments.
Did you know?
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.