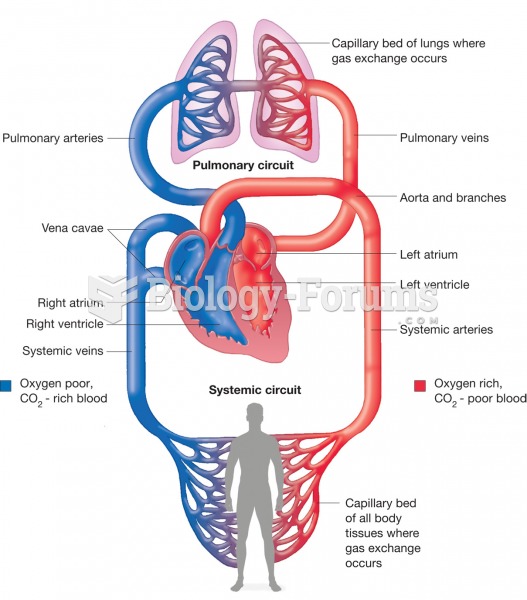
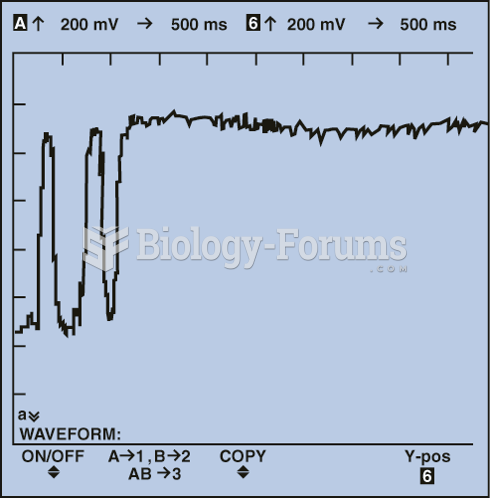
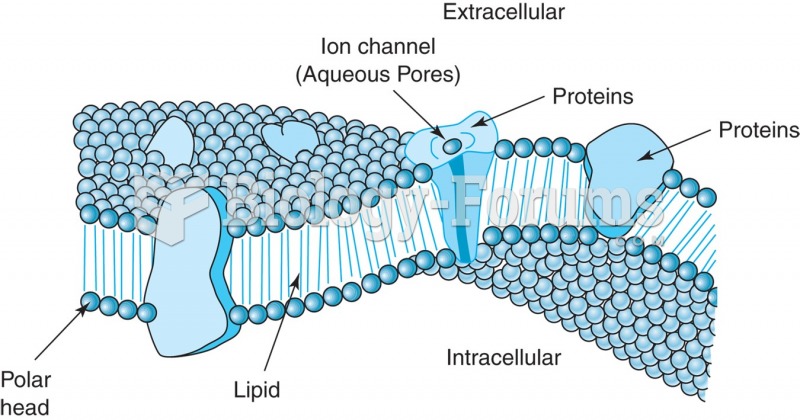
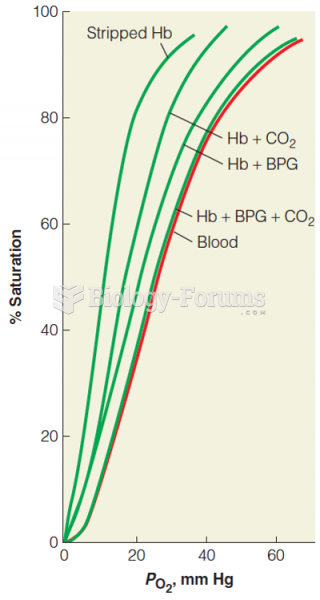
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The FDA recognizes 118 routes of administration.
Did you know?
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
Did you know?
Blastomycosis is often misdiagnosed, resulting in tragic outcomes. It is caused by a fungus living in moist soil, in wooded areas of the United States and Canada. If inhaled, the fungus can cause mild breathing problems that may worsen and cause serious illness and even death.
Did you know?
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.
Did you know?
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.