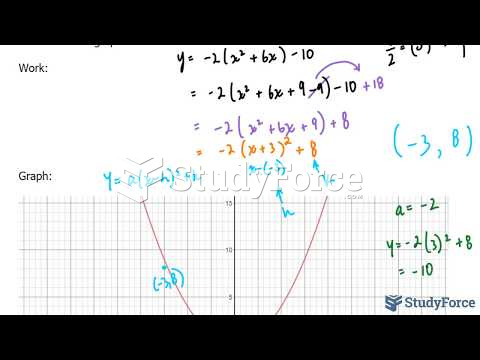
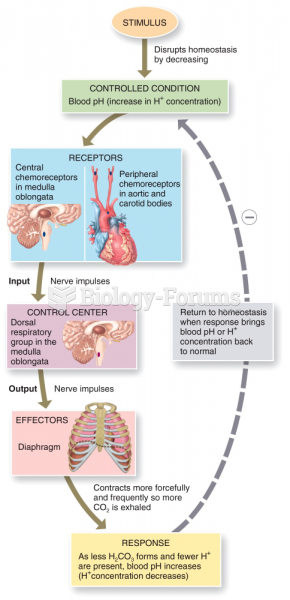
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Patients who have been on total parenteral nutrition for more than a few days may need to have foods gradually reintroduced to give the digestive tract time to start working again.
Did you know?
The newest statin drug, rosuvastatin, has been called a superstatin because it appears to reduce LDL cholesterol to a greater degree than the other approved statin drugs.
Did you know?
Less than one of every three adults with high LDL cholesterol has the condition under control. Only 48.1% with the condition are being treated for it.
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.
Did you know?
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.