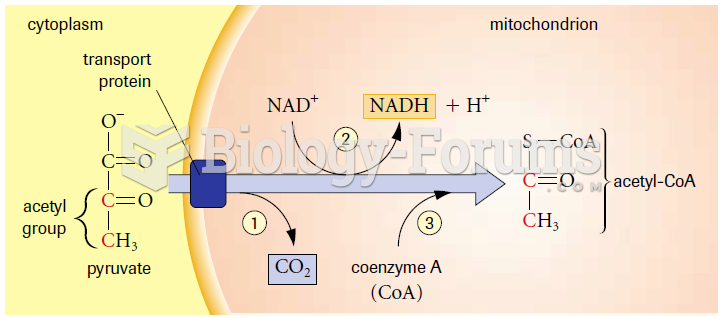
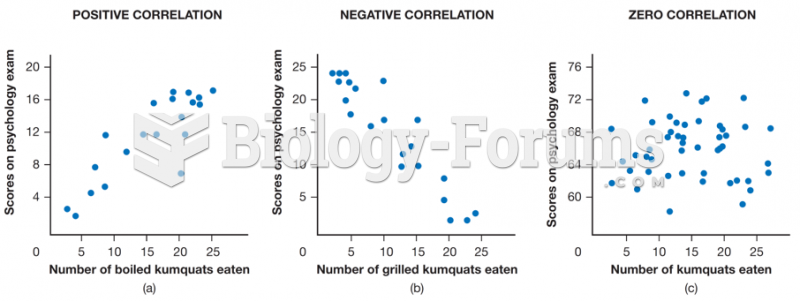
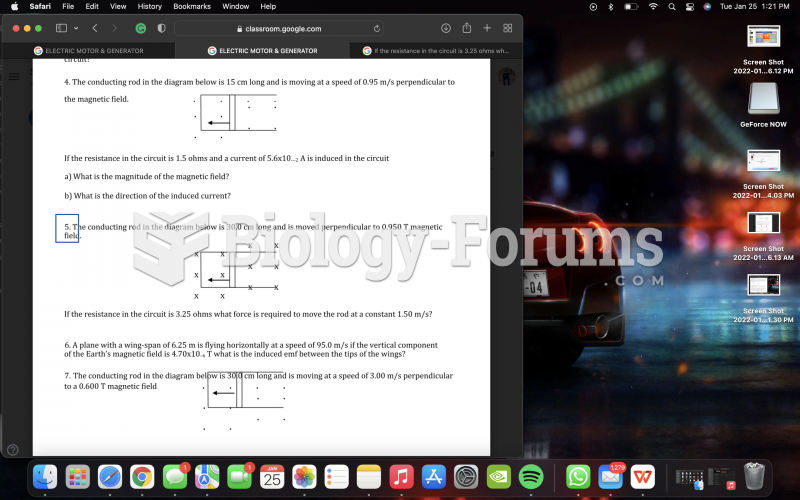
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
Did you know?
Most women experience menopause in their 50s. However, in 1994, an Italian woman gave birth to a baby boy when she was 61 years old.
Did you know?
Autoimmune diseases occur when the immune system destroys its own healthy tissues. When this occurs, white blood cells cannot distinguish between pathogens and normal cells.
Did you know?
One way to reduce acid reflux is to lose two or three pounds. Most people lose weight in the belly area first when they increase exercise, meaning that heartburn can be reduced quickly by this method.
Did you know?
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.