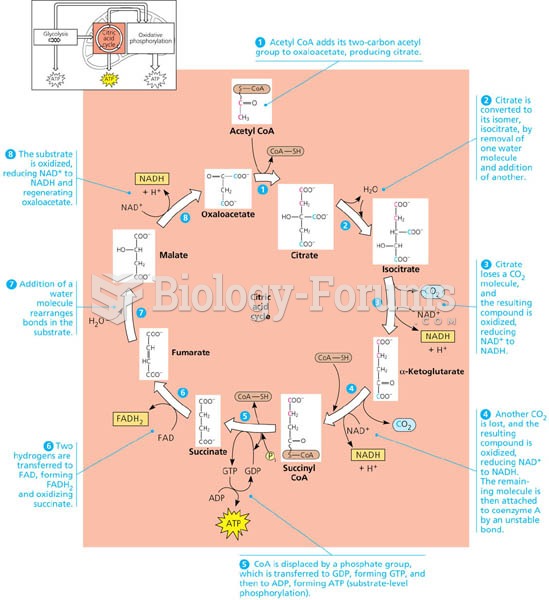
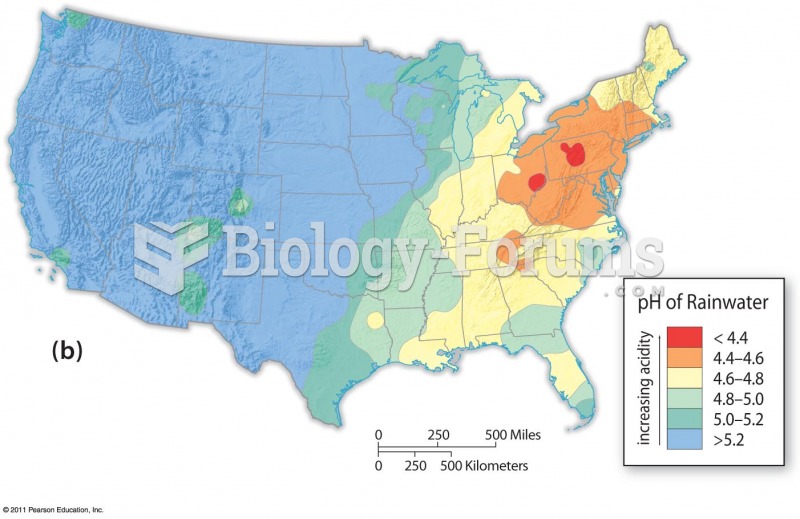
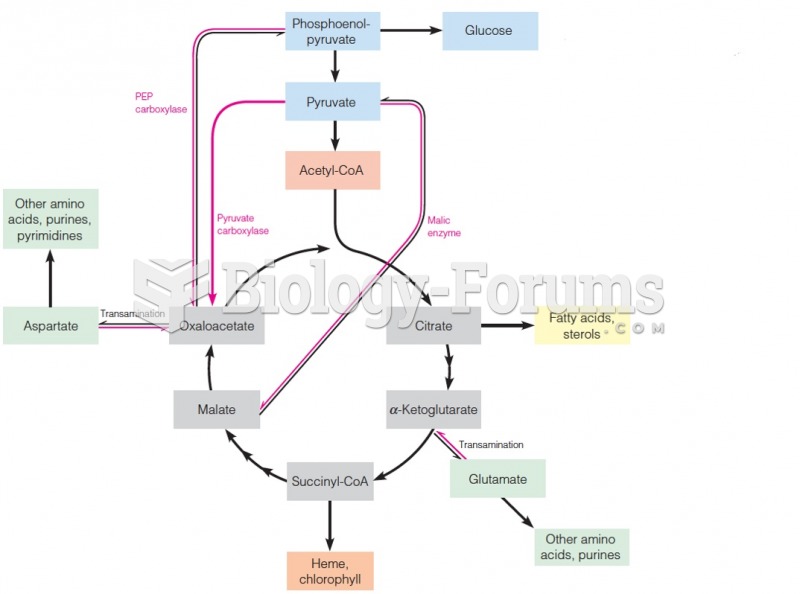
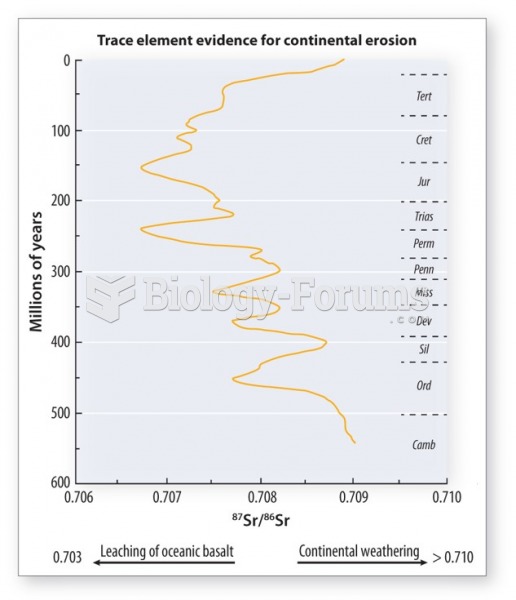
|
|
|
It is important to read food labels and choose foods with low cholesterol and saturated trans fat. You should limit saturated fat to no higher than 6% of daily calories.
In the ancient and medieval periods, dysentery killed about ? of all babies before they reach 12 months of age. The disease was transferred through contaminated drinking water, because there was no way to adequately dispose of sewage, which contaminated the water.
Approximately 500,000 babies are born each year in the United States to teenage mothers.
Sperm cells are so tiny that 400 to 500 million (400,000,000–500,000,000) of them fit onto 1 tsp.
More than 20 million Americans cite use of marijuana within the past 30 days, according to the National Survey on Drug Use and Health (NSDUH). More than 8 million admit to using it almost every day.