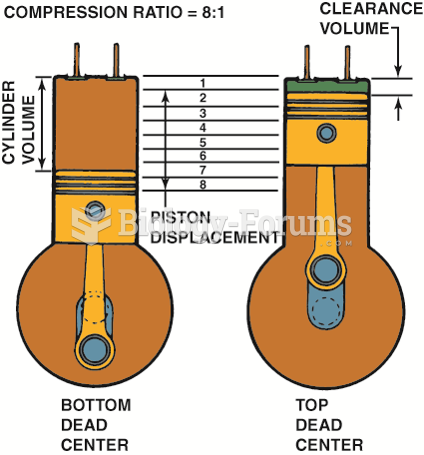
|
|
|
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
Elderly adults are at greatest risk of stroke and myocardial infarction and have the most to gain from prophylaxis. Patients ages 60 to 80 years with blood pressures above 160/90 mm Hg should benefit from antihypertensive treatment.
Alzheimer's disease affects only about 10% of people older than 65 years of age. Most forms of decreased mental function and dementia are caused by disuse (letting the mind get lazy).
As many as 28% of hospitalized patients requiring mechanical ventilators to help them breathe (for more than 48 hours) will develop ventilator-associated pneumonia. Current therapy involves intravenous antibiotics, but new antibiotics that can be inhaled (and more directly treat the infection) are being developed.
Since 1988, the CDC has reported a 99% reduction in bacterial meningitis caused by Haemophilus influenzae, due to the introduction of the vaccine against it.
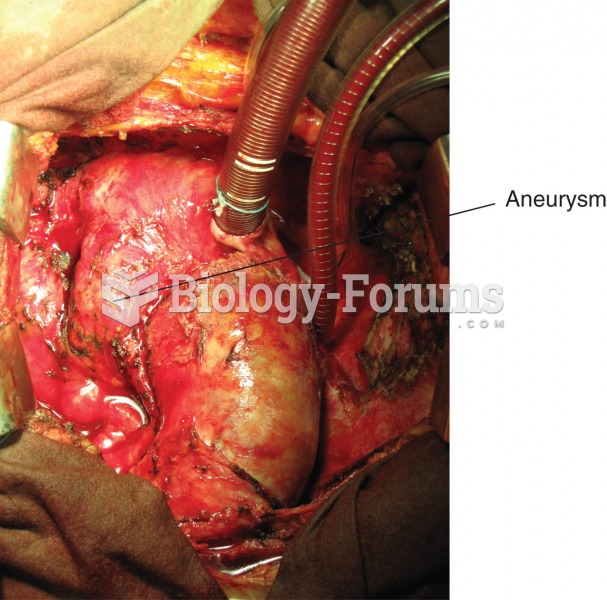 Aneurysm. Photograph of the aorta, the large blood vessel arising from the heart, with a large bulge
Aneurysm. Photograph of the aorta, the large blood vessel arising from the heart, with a large bulge
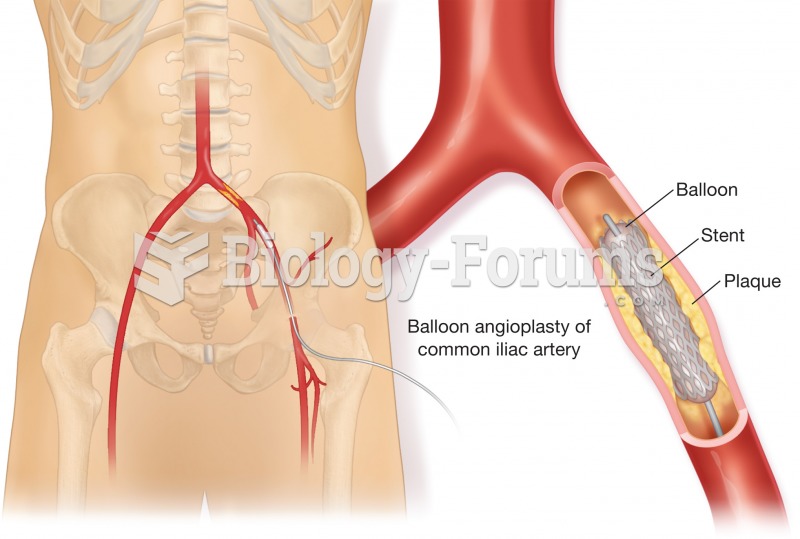 Angioplasty. One form is called balloon angioplasty, shown here. A balloon catheter is threaded into
Angioplasty. One form is called balloon angioplasty, shown here. A balloon catheter is threaded into
 In addition to common schools, thousands of female seminaries were built between 1820 and 1850, many
In addition to common schools, thousands of female seminaries were built between 1820 and 1850, many
 Xenophon (430–357 B.C.E.) Biographer of Socrates and his student as a youth. In addition to four ...
Xenophon (430–357 B.C.E.) Biographer of Socrates and his student as a youth. In addition to four ...