|
|
|
Despite claims by manufacturers, the supplement known as Ginkgo biloba was shown in a study of more than 3,000 participants to be ineffective in reducing development of dementia and Alzheimer’s disease in older people.
Computer programs are available that crosscheck a new drug's possible trade name with all other trade names currently available. These programs detect dangerous similarities between names and alert the manufacturer of the drug.
Walt Disney helped combat malaria by making an animated film in 1943 called The Winged Scourge. This short film starred the seven dwarfs and taught children that mosquitos transmit malaria, which is a very bad disease. It advocated the killing of mosquitos to stop the disease.
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.
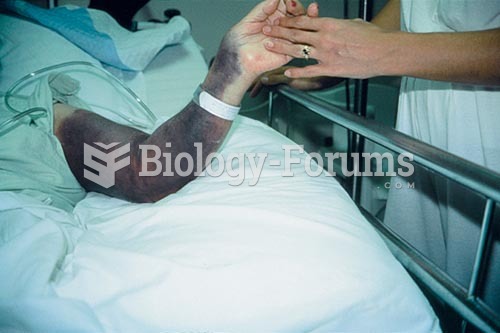 Proper placement and monitoring of an automatic blood pressure cuff will reduce the risk of injury o
Proper placement and monitoring of an automatic blood pressure cuff will reduce the risk of injury o
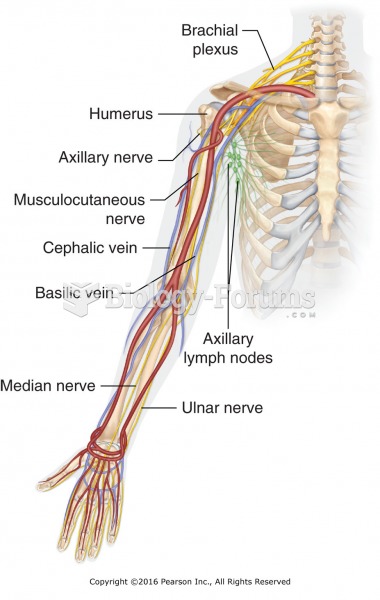 The anterior arm (shoulder, axilla, anticubital, and inner wrist areas). Approach deep pressure in ...
The anterior arm (shoulder, axilla, anticubital, and inner wrist areas). Approach deep pressure in ...