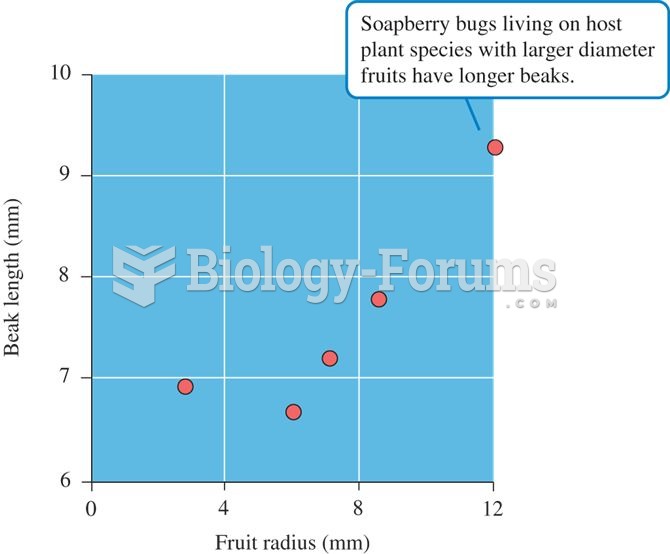
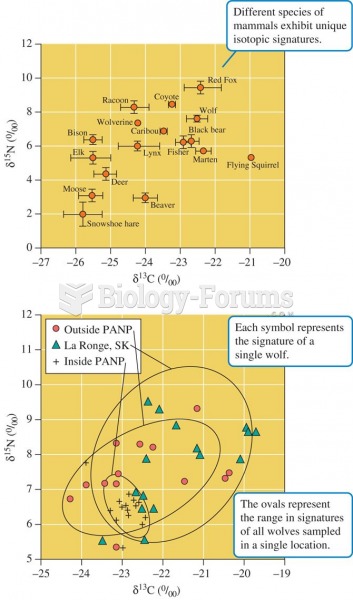
|
|
|
Critical care patients are twice as likely to receive the wrong medication. Of these errors, 20% are life-threatening, and 42% require additional life-sustaining treatments.
Limit intake of red meat and dairy products made with whole milk. Choose skim milk, low-fat or fat-free dairy products. Limit fried food. Use healthy oils when cooking.
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
To maintain good kidney function, you should drink at least 3 quarts of water daily. Water dilutes urine and helps prevent concentrations of salts and minerals that can lead to kidney stone formation. Chronic dehydration is a major contributor to the development of kidney stones.
It is important to read food labels and choose foods with low cholesterol and saturated trans fat. You should limit saturated fat to no higher than 6% of daily calories.