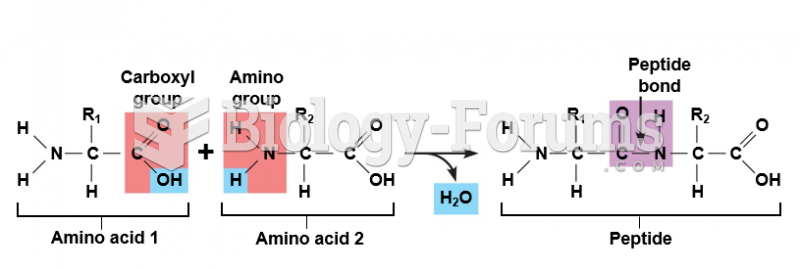
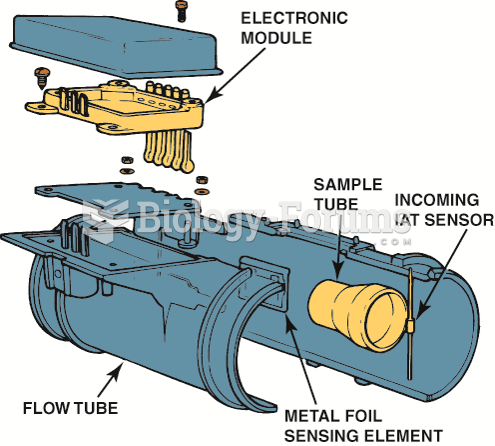
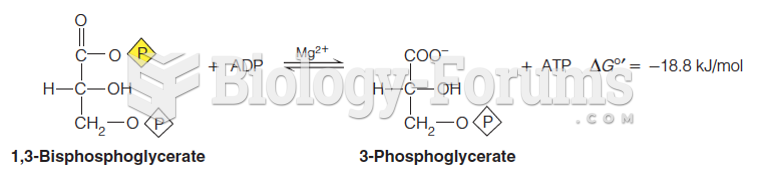
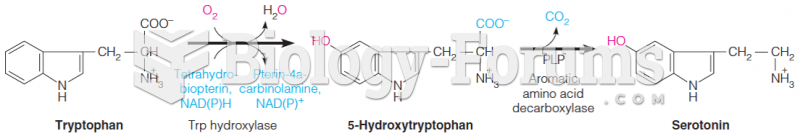
|
|
|
Throughout history, plants containing cardiac steroids have been used as heart drugs and as poisons (e.g., in arrows used in combat), emetics, and diuretics.
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
The Centers for Disease Control and Prevention (CDC) was originally known as the Communicable Disease Center, which was formed to fight malaria. It was originally headquartered in Atlanta, Georgia, since the Southern states faced the worst threat from malaria.