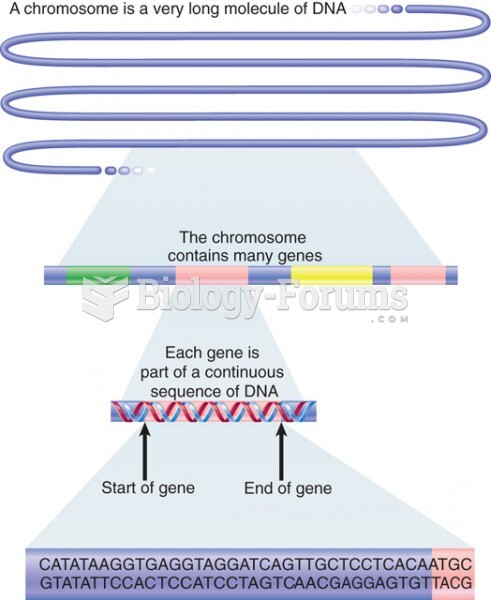
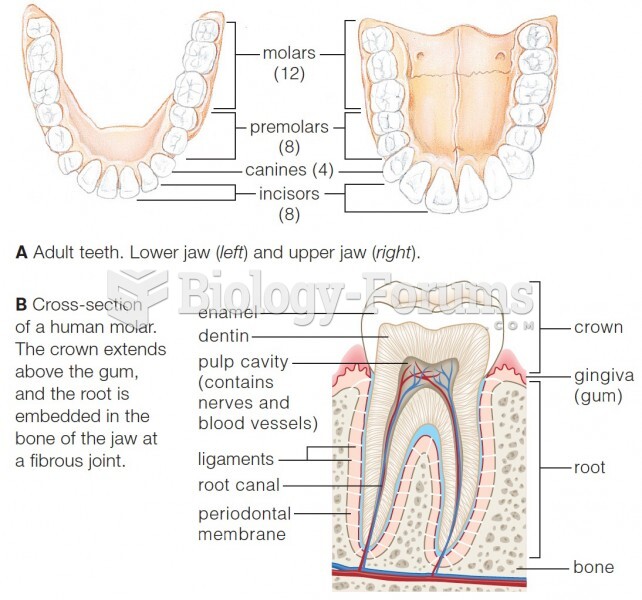
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The types of cancer that alpha interferons are used to treat include hairy cell leukemia, melanoma, follicular non-Hodgkin's lymphoma, and AIDS-related Kaposi's sarcoma.
Did you know?
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.
Did you know?
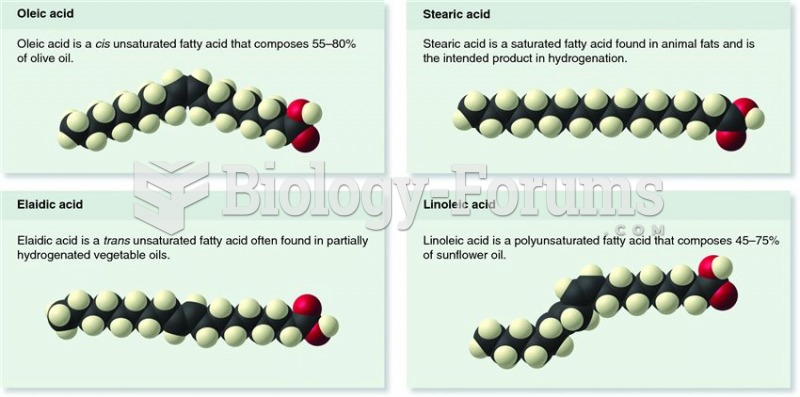
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
Did you know?
Colchicine is a highly poisonous alkaloid originally extracted from a type of saffron plant that is used mainly to treat gout.
Did you know?
It is widely believed that giving a daily oral dose of aspirin to heart attack patients improves their chances of survival because the aspirin blocks the formation of new blood clots.