|
|
|
Green tea is able to stop the scent of garlic or onion from causing bad breath.
Asthma is the most common chronic childhood disease in the world. Most children who develop asthma have symptoms before they are 5 years old.
Approximately one in four people diagnosed with diabetes will develop foot problems. Of these, about one-third will require lower extremity amputation.
The eye muscles are the most active muscles in the whole body. The external muscles that move the eyes are the strongest muscles in the human body for the job they have to do. They are 100 times more powerful than they need to be.
For about 100 years, scientists thought that peptic ulcers were caused by stress, spicy food, and alcohol. Later, researchers added stomach acid to the list of causes and began treating ulcers with antacids. Now it is known that peptic ulcers are predominantly caused by Helicobacter pylori, a spiral-shaped bacterium that normally exist in the stomach.
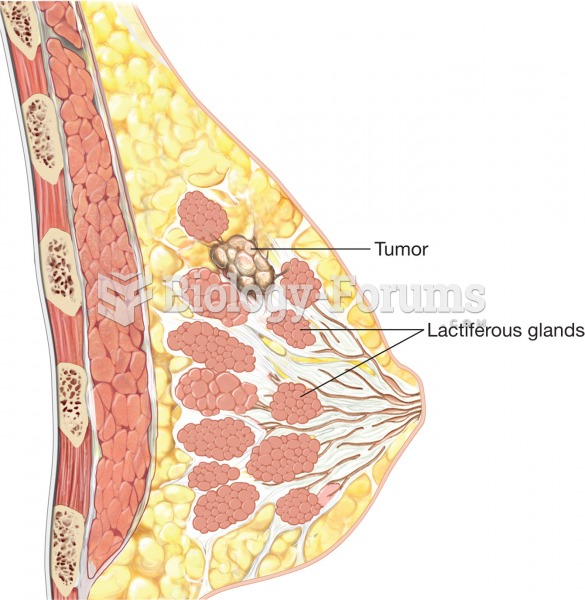 Breast cancer. Notice the tumor growing within a lactiferous gland, which occurs in infiltrating duc
Breast cancer. Notice the tumor growing within a lactiferous gland, which occurs in infiltrating duc
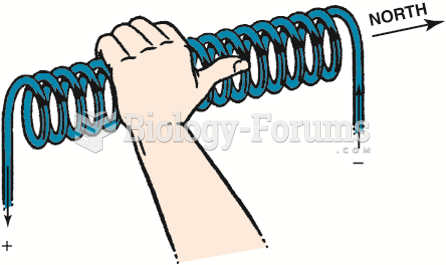 The left-hand rule states that if a coil is grasped with the left hand, the fingers will point in ...
The left-hand rule states that if a coil is grasped with the left hand, the fingers will point in ...