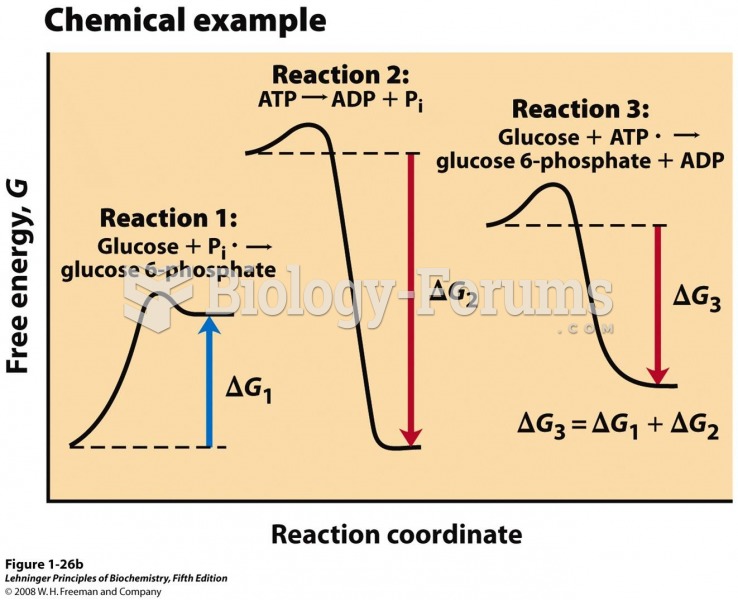
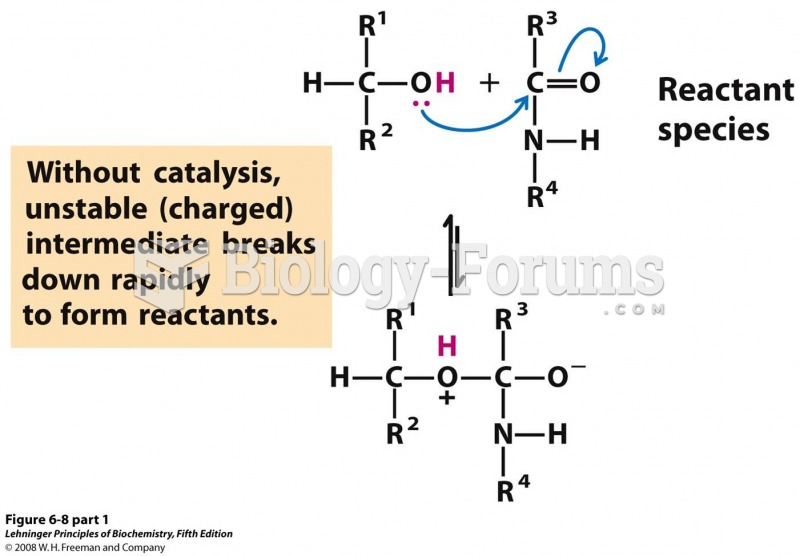
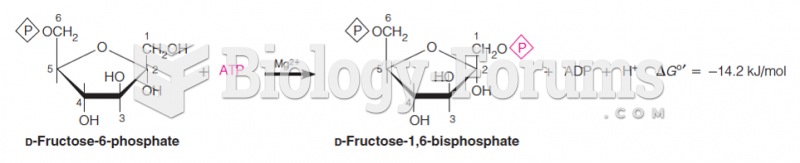
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.
Did you know?
Signs of depression include feeling sad most of the time for 2 weeks or longer; loss of interest in things normally enjoyed; lack of energy; sleep and appetite disturbances; weight changes; feelings of hopelessness, helplessness, or worthlessness; an inability to make decisions; and thoughts of death and suicide.
Did you know?
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
Did you know?
About 600,000 particles of skin are shed every hour by each human. If you live to age 70 years, you have shed 105 pounds of dead skin.