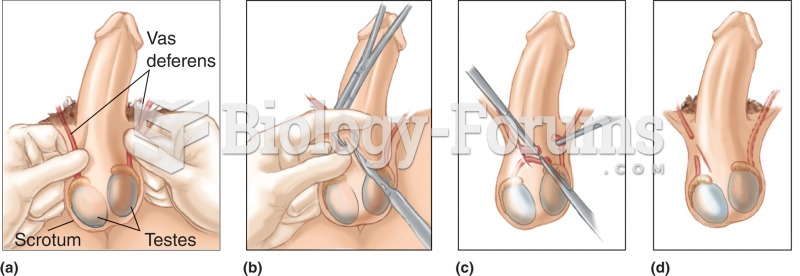
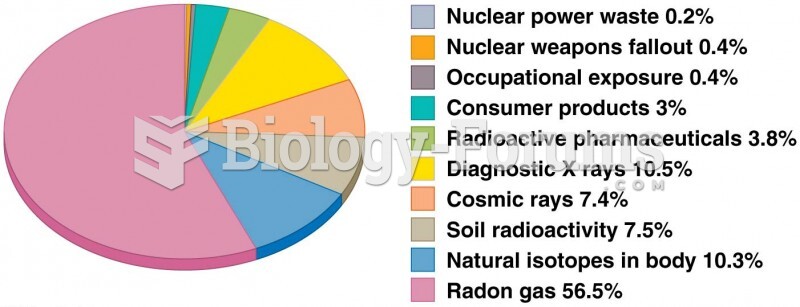
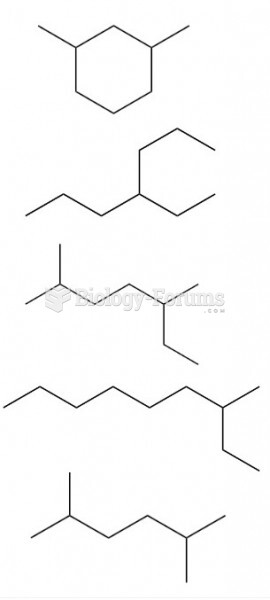
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
More than one-third of adult Americans are obese. Diseases that kill the largest number of people annually, such as heart disease, cancer, diabetes, stroke, and hypertension, can be attributed to diet.
Did you know?
Blood is approximately twice as thick as water because of the cells and other components found in it.
Did you know?
Acetaminophen (Tylenol) in overdose can seriously damage the liver. It should never be taken by people who use alcohol heavily; it can result in severe liver damage and even a condition requiring a liver transplant.
Did you know?
Excessive alcohol use costs the country approximately $235 billion every year.
Did you know?
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.