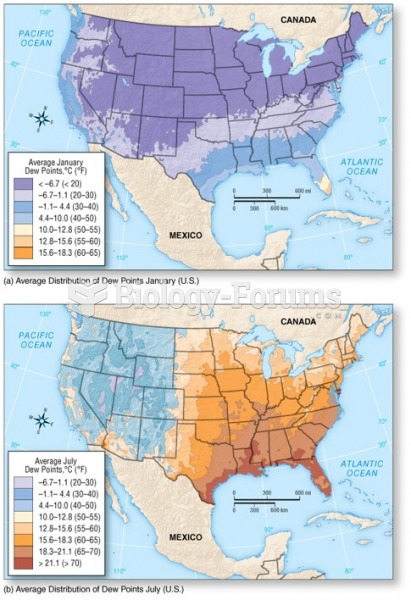
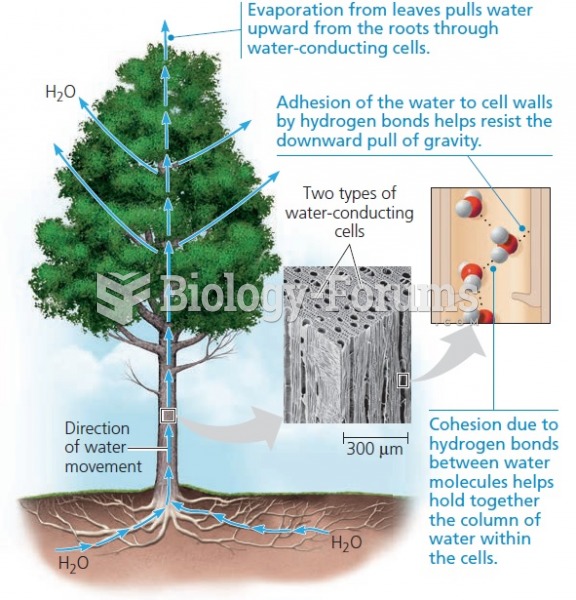
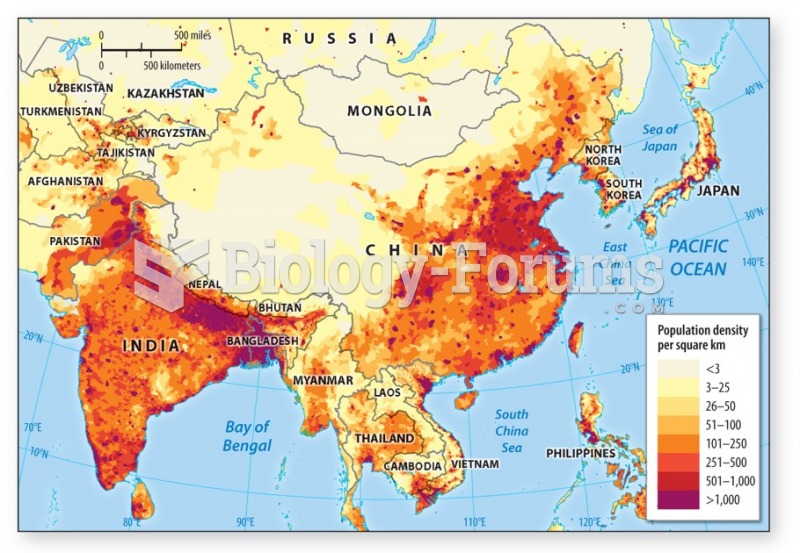
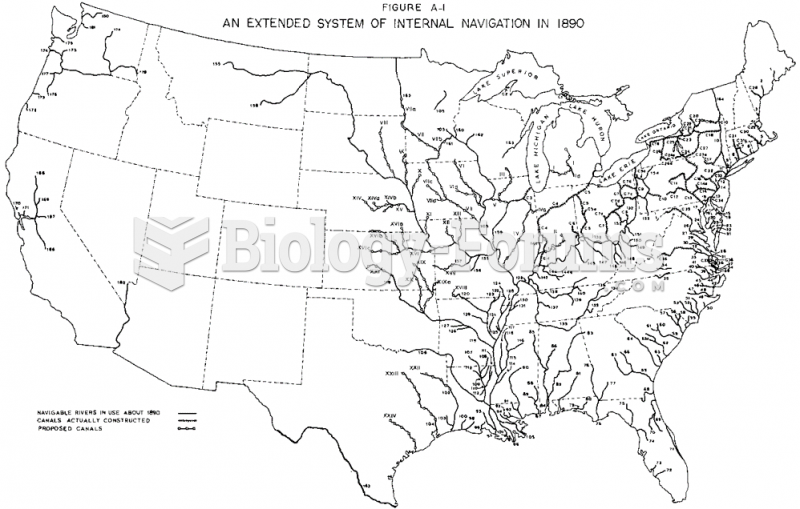
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Did you know?
The horizontal fraction bar was introduced by the Arabs.
Did you know?
Allergies play a major part in the health of children. The most prevalent childhood allergies are milk, egg, soy, wheat, peanuts, tree nuts, and seafood.
Did you know?
Approximately 15–25% of recognized pregnancies end in miscarriage. However, many miscarriages often occur before a woman even knows she is pregnant.
Did you know?
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.