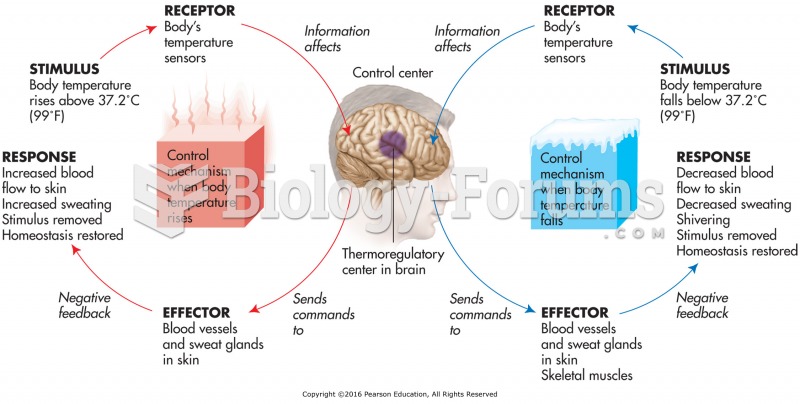
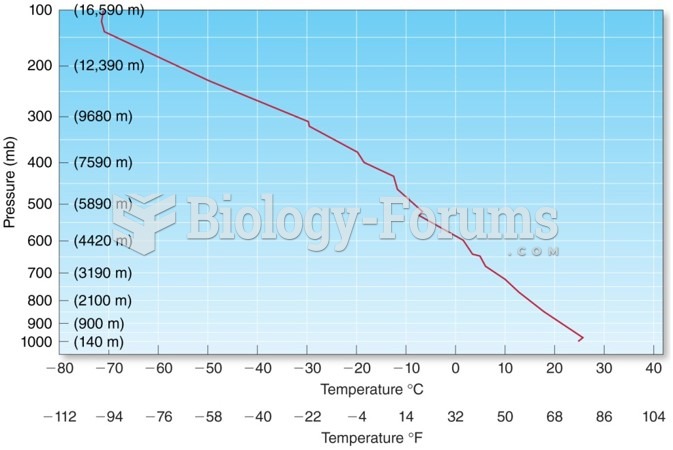
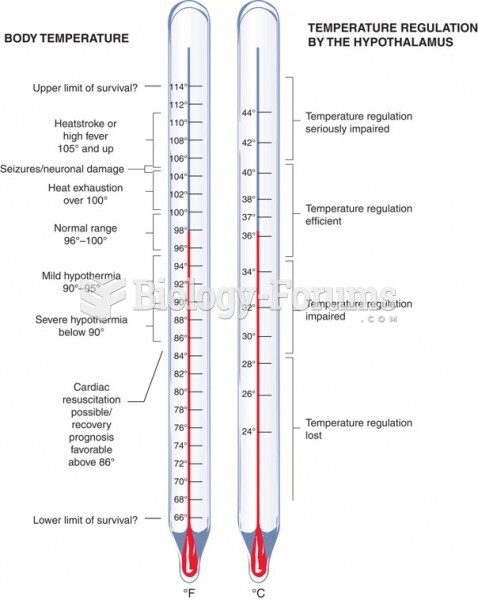
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Patients should never assume they are being given the appropriate drugs. They should make sure they know which drugs are being prescribed, and always double-check that the drugs received match the prescription.
Did you know?
Atropine, along with scopolamine and hyoscyamine, is found in the Datura stramonium plant, which gives hallucinogenic effects and is also known as locoweed.
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.
Did you know?
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.