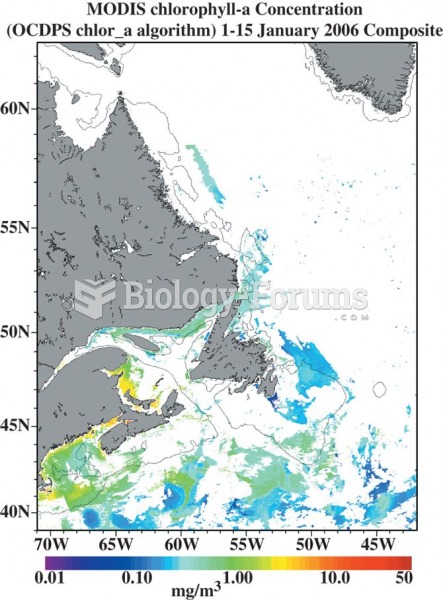
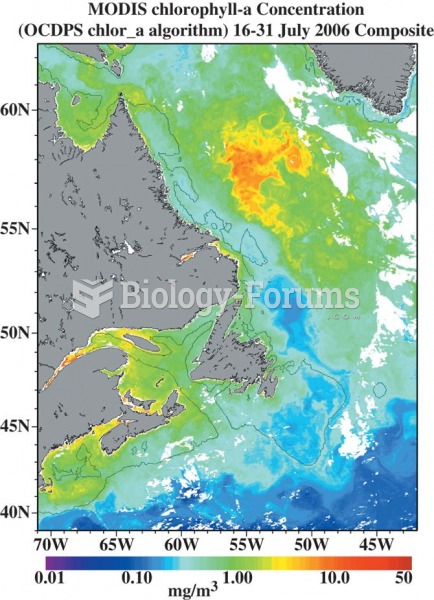
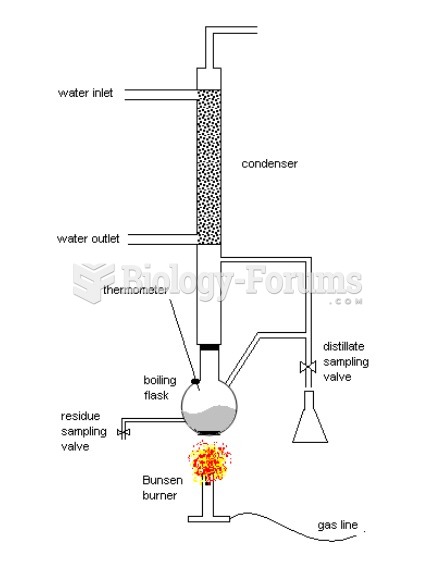
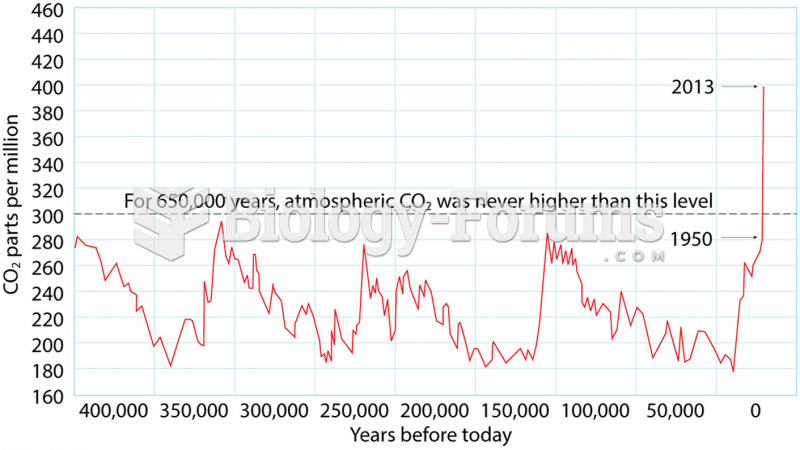
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.
Did you know?
Symptoms of kidney problems include a loss of appetite, back pain (which may be sudden and intense), chills, abdominal pain, fluid retention, nausea, the urge to urinate, vomiting, and fever.
Did you know?
One way to reduce acid reflux is to lose two or three pounds. Most people lose weight in the belly area first when they increase exercise, meaning that heartburn can be reduced quickly by this method.
Did you know?
The heart is located in the center of the chest, with part of it tipped slightly so that it taps against the left side of the chest.
Did you know?
The people with the highest levels of LDL are Mexican American males and non-Hispanic black females.