|
|
|
Children with strabismus (crossed eyes) can be treated. They are not able to outgrow this condition on their own, but with help, it can be more easily corrected at a younger age. It is important for infants to have eye examinations as early as possible in their development and then another at age 2 years.
Asthma cases in Americans are about 75% higher today than they were in 1980.
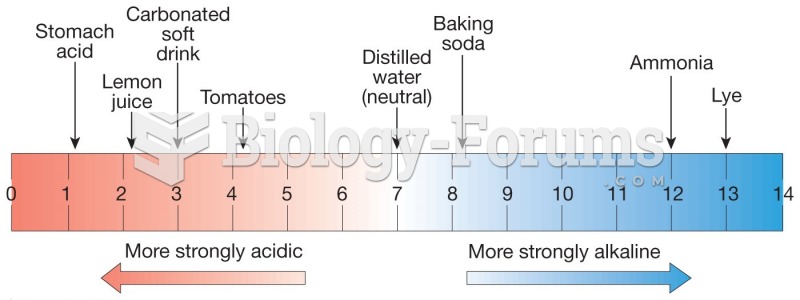
For about 100 years, scientists thought that peptic ulcers were caused by stress, spicy food, and alcohol. Later, researchers added stomach acid to the list of causes and began treating ulcers with antacids. Now it is known that peptic ulcers are predominantly caused by Helicobacter pylori, a spiral-shaped bacterium that normally exist in the stomach.
In most cases, kidneys can recover from almost complete loss of function, such as in acute kidney (renal) failure.
Cocaine was isolated in 1860 and first used as a local anesthetic in 1884. Its first clinical use was by Sigmund Freud to wean a patient from morphine addiction. The fictional character Sherlock Holmes was supposed to be addicted to cocaine by injection.