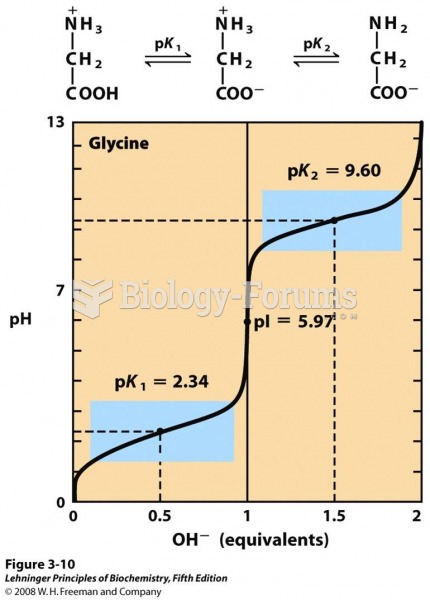
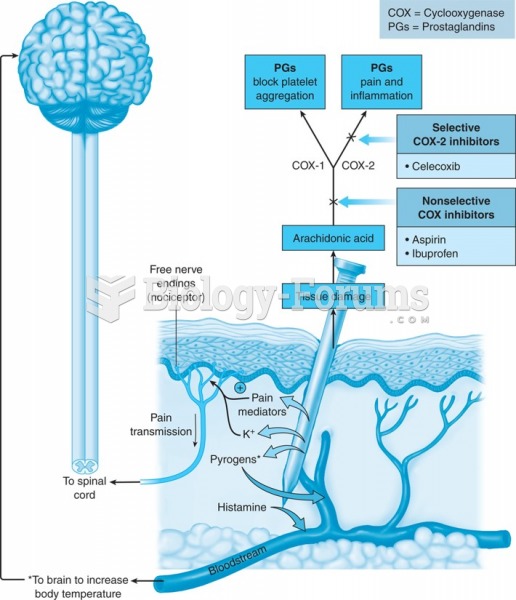
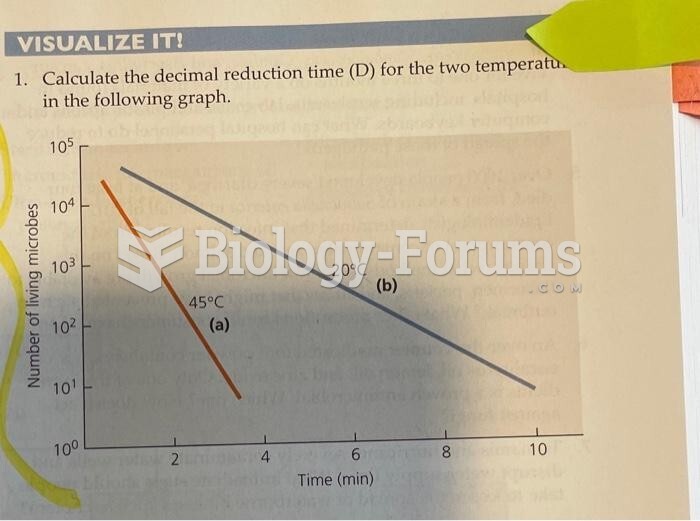
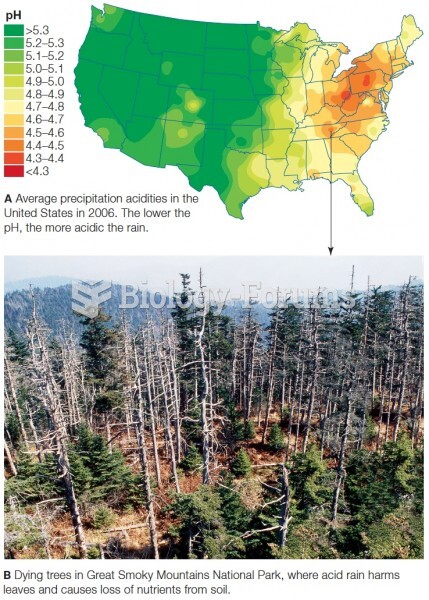
|
|
|
More than 150,000 Americans killed by cardiovascular disease are younger than the age of 65 years.
Medications that are definitely not safe to take when breastfeeding include radioactive drugs, antimetabolites, some cancer (chemotherapy) agents, bromocriptine, ergotamine, methotrexate, and cyclosporine.
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
Certain topical medications such as clotrimazole and betamethasone are not approved for use in children younger than 12 years of age. They must be used very cautiously, as directed by a doctor, to treat any child. Children have a much greater response to topical steroid medications.