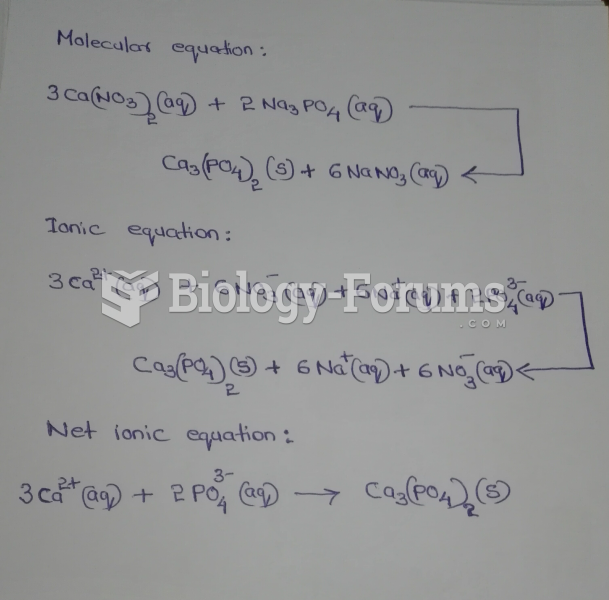
|
|
|
Fungal nail infections account for up to 30% of all skin infections. They affect 5% of the general population—mostly people over the age of 70.
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
Medication errors are more common among seriously ill patients than with those with minor conditions.
Warfarin was developed as a consequence of the study of a strange bleeding disorder that suddenly occurred in cattle on the northern prairies of the United States in the early 1900s.
People often find it difficult to accept the idea that bacteria can be beneficial and improve health. Lactic acid bacteria are good, and when eaten, these bacteria improve health and increase longevity. These bacteria included in foods such as yogurt.


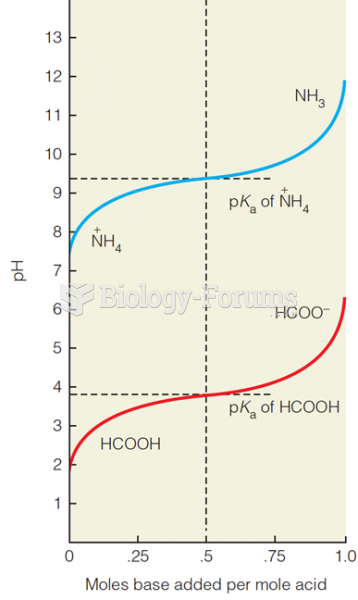

![What is the pH of an aqueous solution if the [H+] = 0.000 000 075 M?](https://biology-forums.com/gallery/43/medium_6_11_12_21_7_23_55.jpeg)