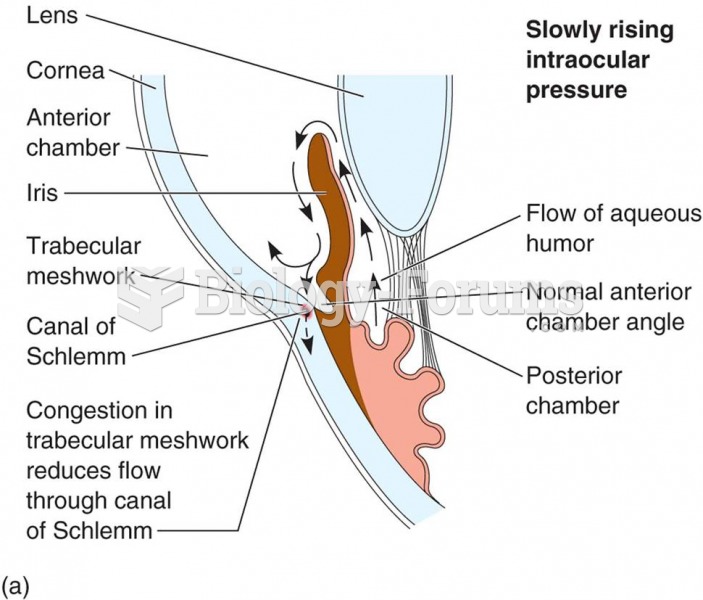
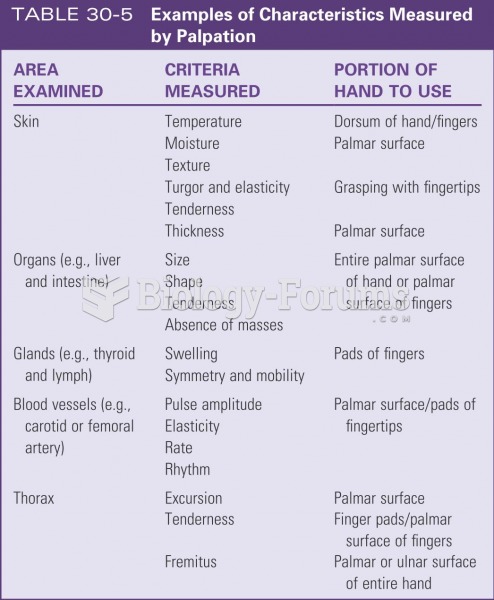
|
|
|
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.
Critical care patients are twice as likely to receive the wrong medication. Of these errors, 20% are life-threatening, and 42% require additional life-sustaining treatments.
The familiar sounds of your heart are made by the heart's valves as they open and close.
Though newer “smart” infusion pumps are increasingly becoming more sophisticated, they cannot prevent all programming and administration errors. Health care professionals that use smart infusion pumps must still practice the rights of medication administration and have other professionals double-check all high-risk infusions.
More than 4.4billion prescriptions were dispensed within the United States in 2016.