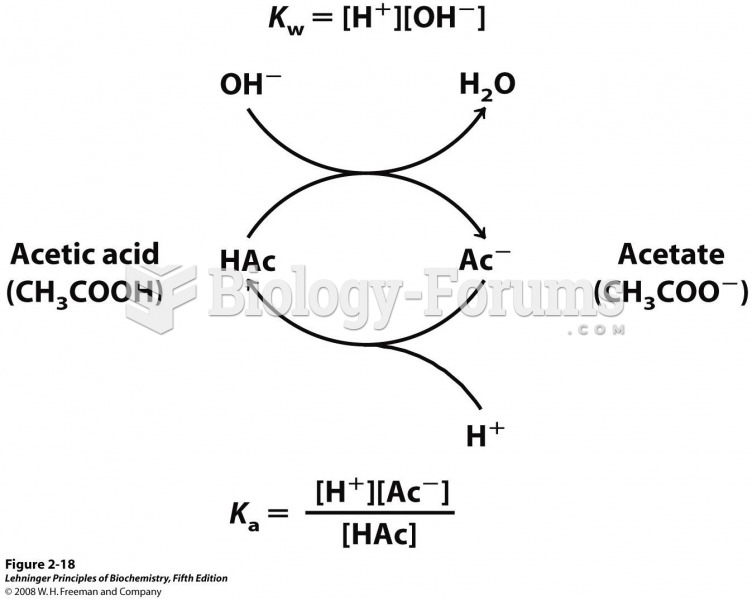
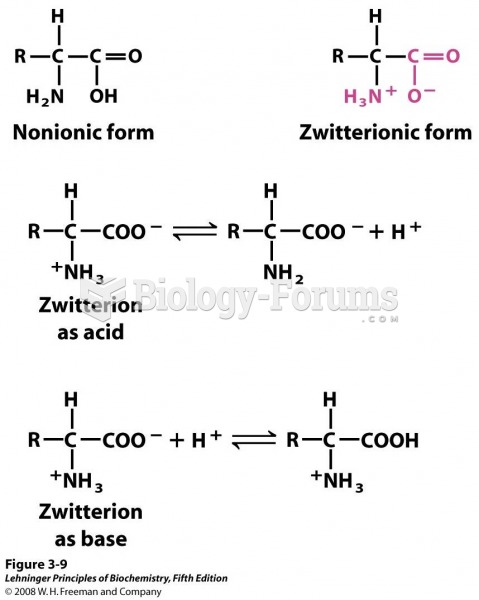
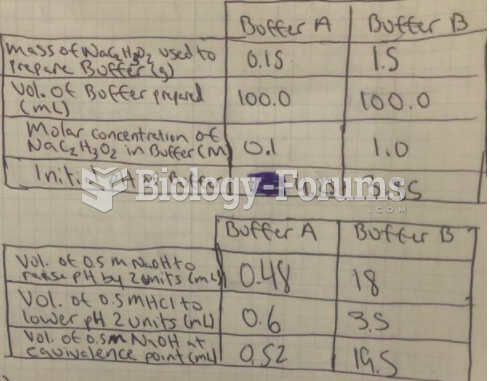
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Sperm cells are so tiny that 400 to 500 million (400,000,000–500,000,000) of them fit onto 1 tsp.
Did you know?
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
Did you know?
Though the United States has largely rejected the metric system, it is used for currency, as in 100 pennies = 1 dollar. Previously, the British currency system was used, with measurements such as 12 pence to the shilling, and 20 shillings to the pound.
Did you know?
The average human gut is home to perhaps 500 to 1,000 different species of bacteria.
Did you know?
Women are 50% to 75% more likely than men to experience an adverse drug reaction.