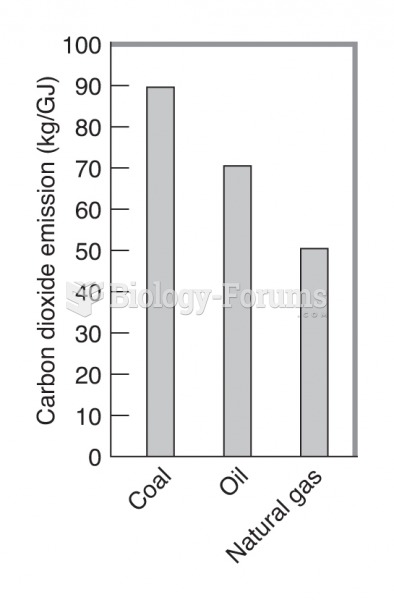
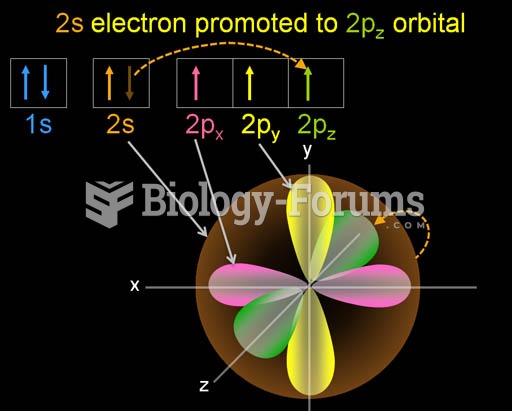
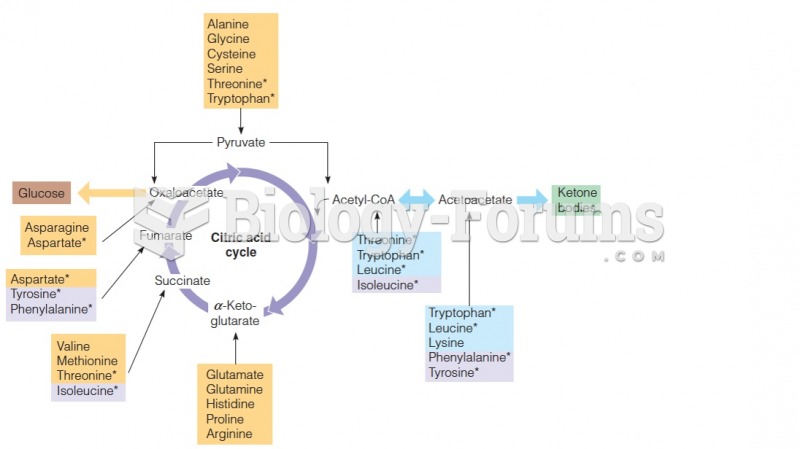
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Sperm cells are so tiny that 400 to 500 million (400,000,000–500,000,000) of them fit onto 1 tsp.
Did you know?
Normal urine is sterile. It contains fluids, salts, and waste products. It is free of bacteria, viruses, and fungi.
Did you know?
In the United States, congenital cytomegalovirus causes one child to become disabled almost every hour. CMV is the leading preventable viral cause of development disability in newborns. These disabilities include hearing or vision loss, and cerebral palsy.
Did you know?
It is difficult to obtain enough calcium without consuming milk or other dairy foods.
Did you know?
The people with the highest levels of LDL are Mexican American males and non-Hispanic black females.