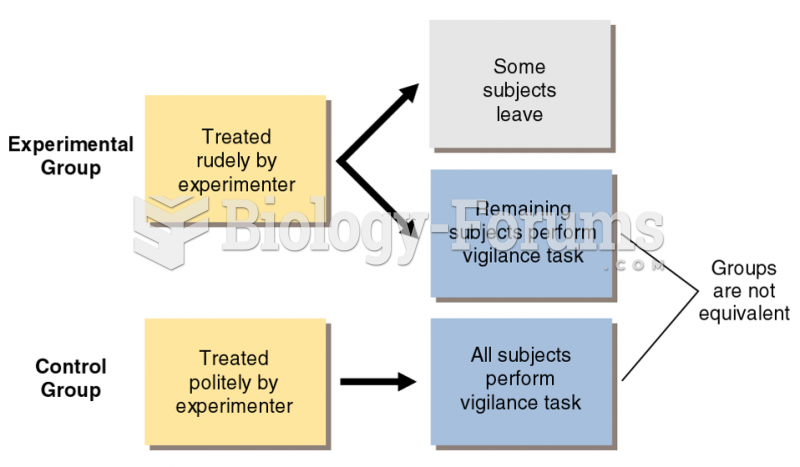
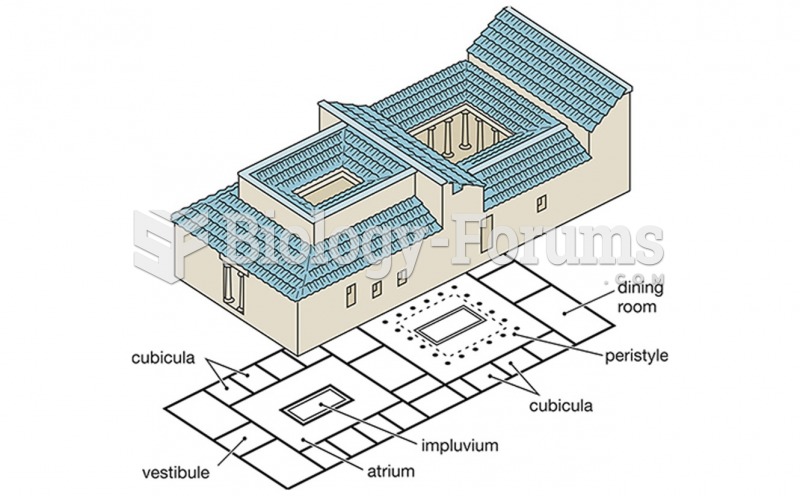
|
|
|
Elderly adults are at greatest risk of stroke and myocardial infarction and have the most to gain from prophylaxis. Patients ages 60 to 80 years with blood pressures above 160/90 mm Hg should benefit from antihypertensive treatment.
The modern decimal position system was the invention of the Hindus (around 800 AD), involving the placing of numerals to indicate their value (units, tens, hundreds, and so on).
All patients with hyperparathyroidism will develop osteoporosis. The parathyroid glands maintain blood calcium within the normal range. All patients with this disease will continue to lose calcium from their bones every day, and there is no way to prevent the development of osteoporosis as a result.
It is believed that the Incas used anesthesia. Evidence supports the theory that shamans chewed cocoa leaves and drilled holes into the heads of patients (letting evil spirits escape), spitting into the wounds they made. The mixture of cocaine, saliva, and resin numbed the site enough to allow hours of drilling.
Multiple sclerosis is a condition wherein the body's nervous system is weakened by an autoimmune reaction that attacks the myelin sheaths of neurons.