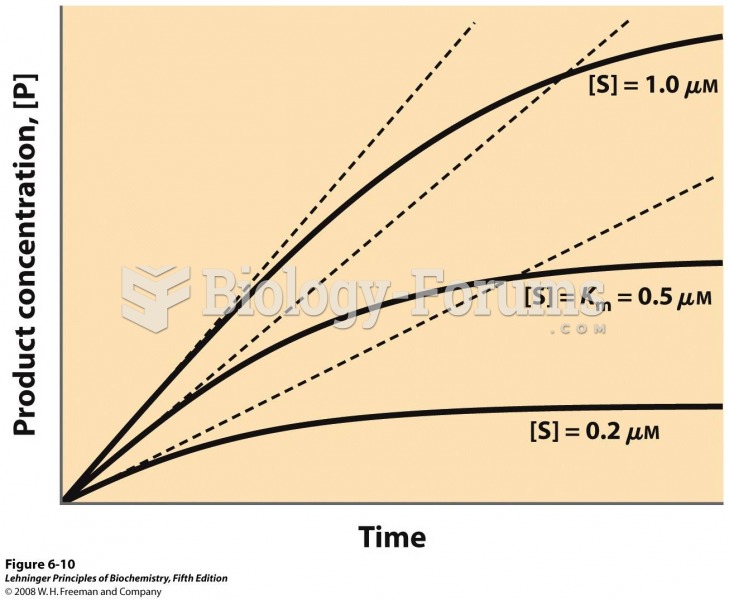
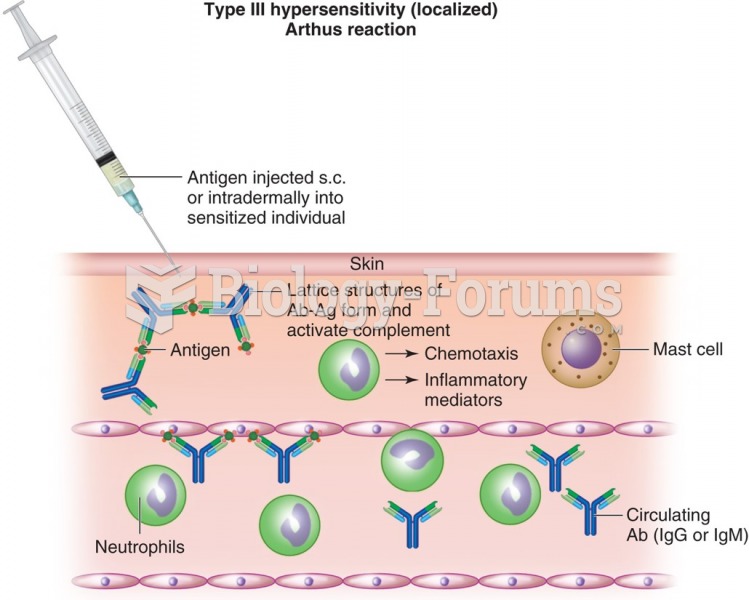
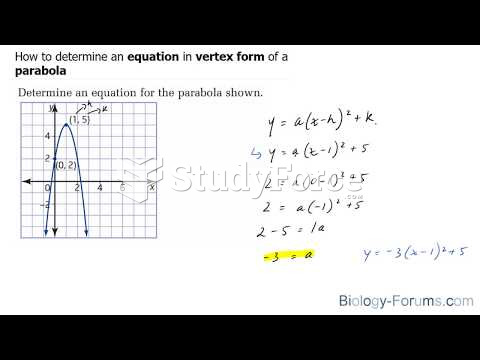
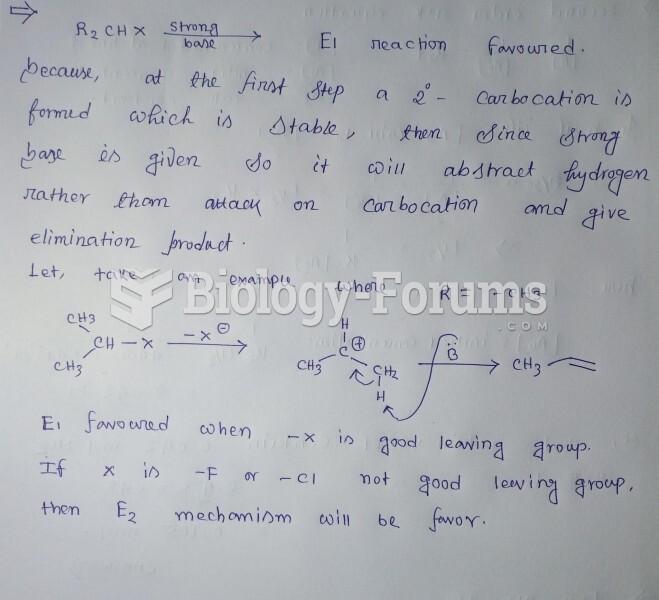
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
When blood is exposed to air, it clots. Heparin allows the blood to come in direct contact with air without clotting.
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.
Did you know?
Hyperthyroidism leads to an increased rate of metabolism and affects about 1% of women but only 0.1% of men. For most people, this increased metabolic rate causes the thyroid gland to become enlarged (known as a goiter).
Did you know?
Fewer than 10% of babies are born on their exact due dates, 50% are born within 1 week of the due date, and 90% are born within 2 weeks of the date.
Did you know?
Today, nearly 8 out of 10 pregnant women living with HIV (about 1.1 million), receive antiretrovirals.