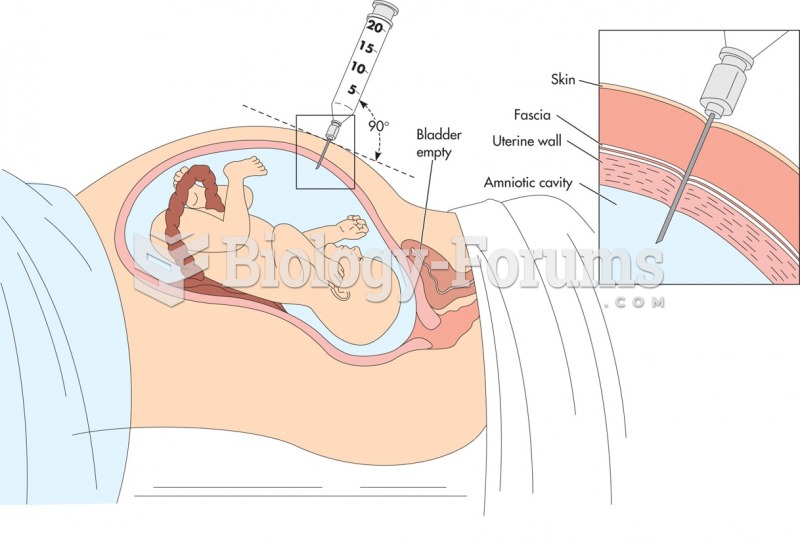
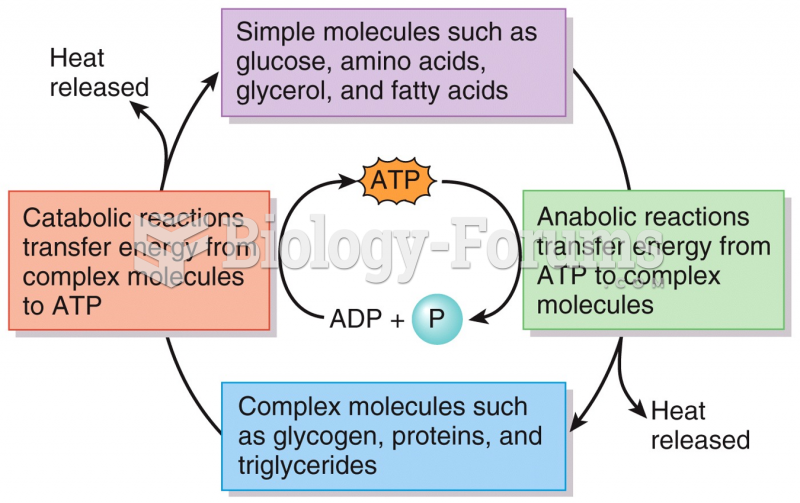
|
|
|
According to the National Institute of Environmental Health Sciences, lung disease is the third leading killer in the United States, responsible for one in seven deaths. It is the leading cause of death among infants under the age of one year.
As the western states of America were settled, pioneers often had to drink rancid water from ponds and other sources. This often resulted in chronic diarrhea, causing many cases of dehydration and death that could have been avoided if clean water had been available.
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
Everyone has one nostril that is larger than the other.