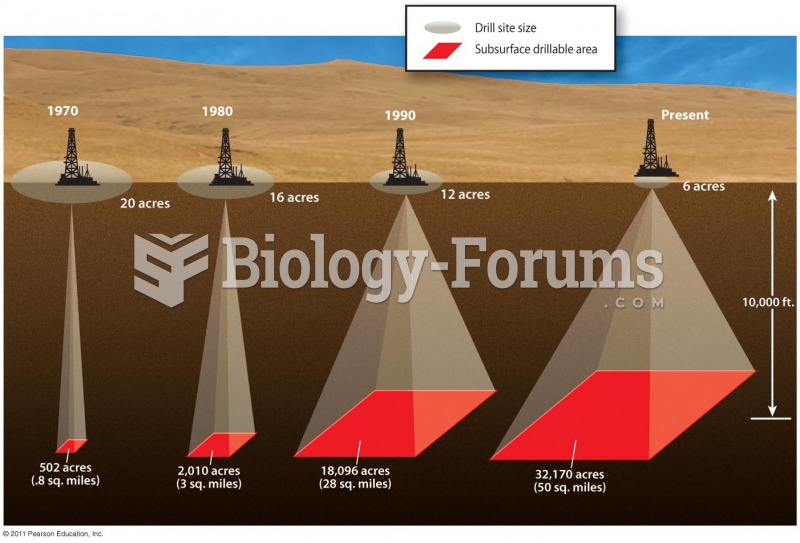
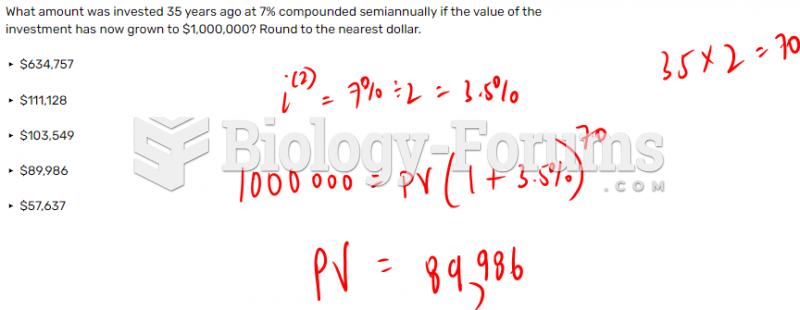
|
|
|
Certain rare plants containing cyanide include apricot pits and a type of potato called cassava. Fortunately, only chronic or massive ingestion of any of these plants can lead to serious poisoning.
The first war in which wide-scale use of anesthetics occurred was the Civil War, and 80% of all wounds were in the extremities.
Amoebae are the simplest type of protozoans, and are characterized by a feeding and dividing trophozoite stage that moves by temporary extensions called pseudopodia or false feet.
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
It is believed that humans initially contracted crabs from gorillas about 3 million years ago from either sleeping in gorilla nests or eating the apes.