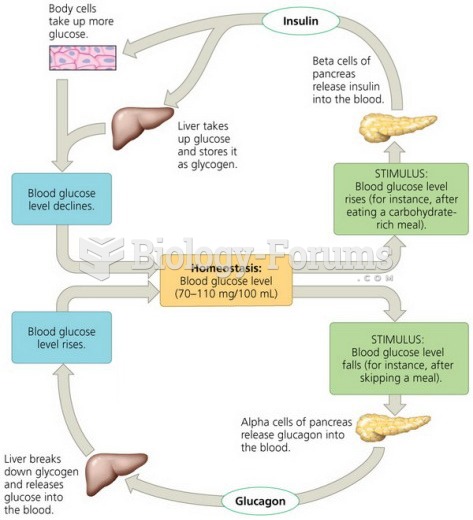
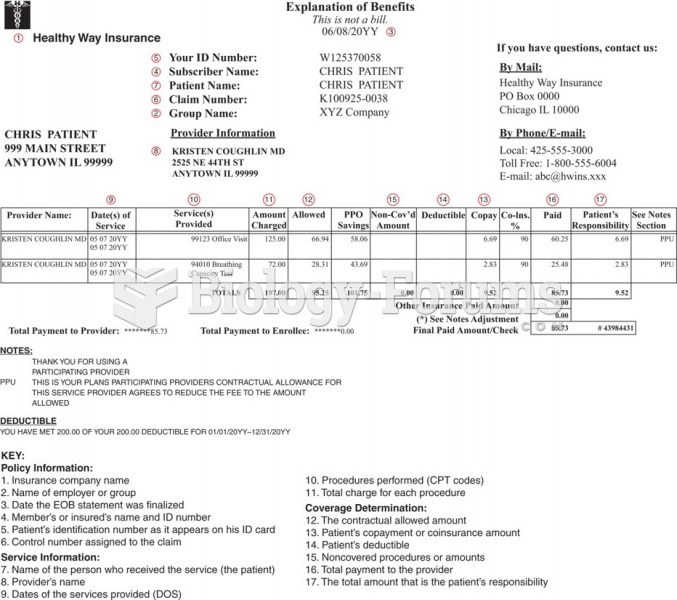
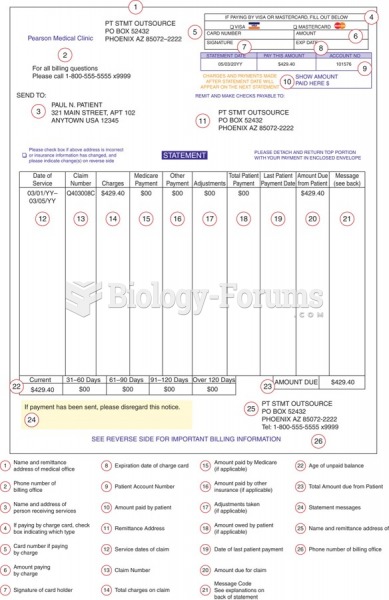
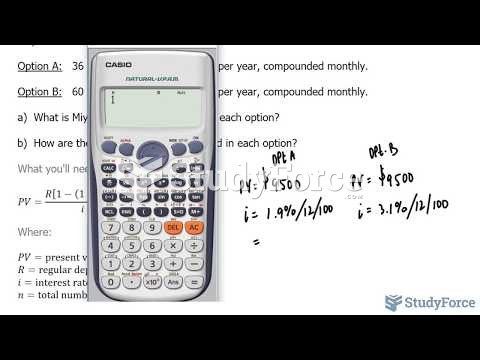
|
|
|
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.
Each year in the United States, there are approximately six million pregnancies. This means that at any one time, about 4% of women in the United States are pregnant.
Atropine, along with scopolamine and hyoscyamine, is found in the Datura stramonium plant, which gives hallucinogenic effects and is also known as locoweed.
Only one in 10 cancer deaths is caused by the primary tumor. The vast majority of cancer mortality is caused by cells breaking away from the main tumor and metastasizing to other parts of the body, such as the brain, bones, or liver.