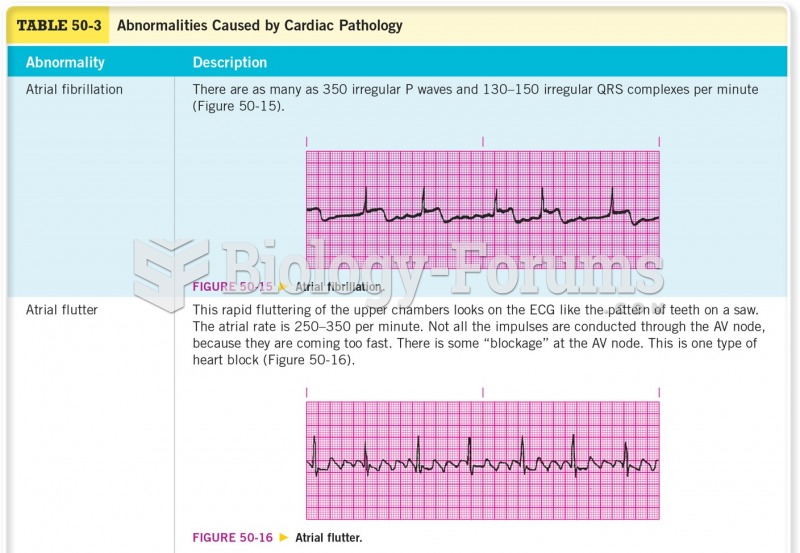
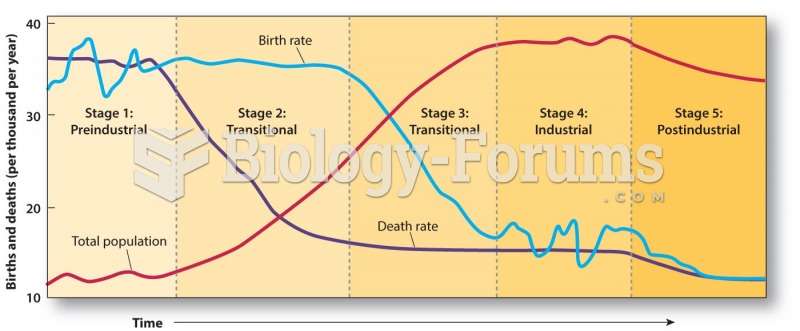
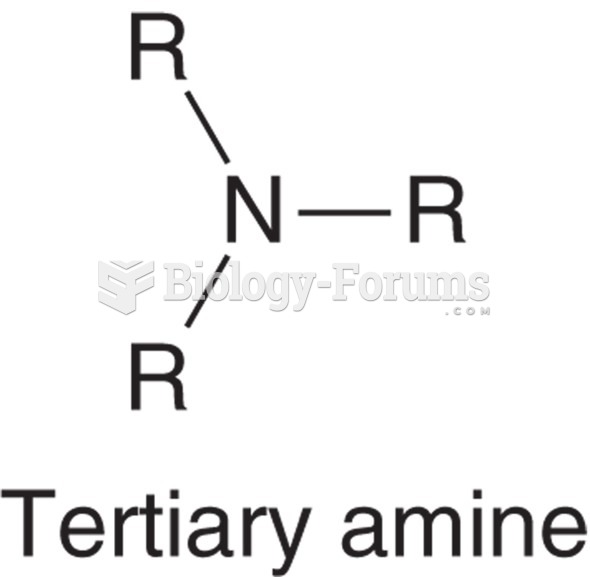
|
|
|
The human body's pharmacokinetics are quite varied. Our hair holds onto drugs longer than our urine, blood, or saliva. For example, alcohol can be detected in the hair for up to 90 days after it was consumed. The same is true for marijuana, cocaine, ecstasy, heroin, methamphetamine, and nicotine.
Throughout history, plants containing cardiac steroids have been used as heart drugs and as poisons (e.g., in arrows used in combat), emetics, and diuretics.
Today, nearly 8 out of 10 pregnant women living with HIV (about 1.1 million), receive antiretrovirals.
The shortest mature adult human of whom there is independent evidence was Gul Mohammed in India. In 1990, he was measured in New Delhi and stood 22.5 inches tall.
Eating carrots will improve your eyesight. Carrots are high in vitamin A (retinol), which is essential for good vision. It can also be found in milk, cheese, egg yolks, and liver.
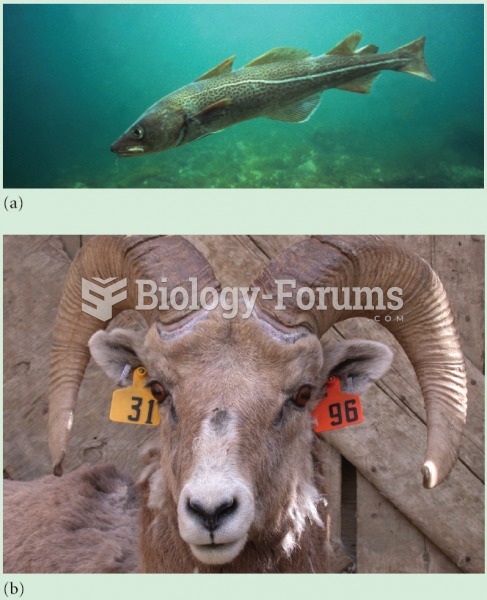 Continued harvest of (a) cod and (b) mountain sheep by humans has caused evolutionary changes in mat
Continued harvest of (a) cod and (b) mountain sheep by humans has caused evolutionary changes in mat
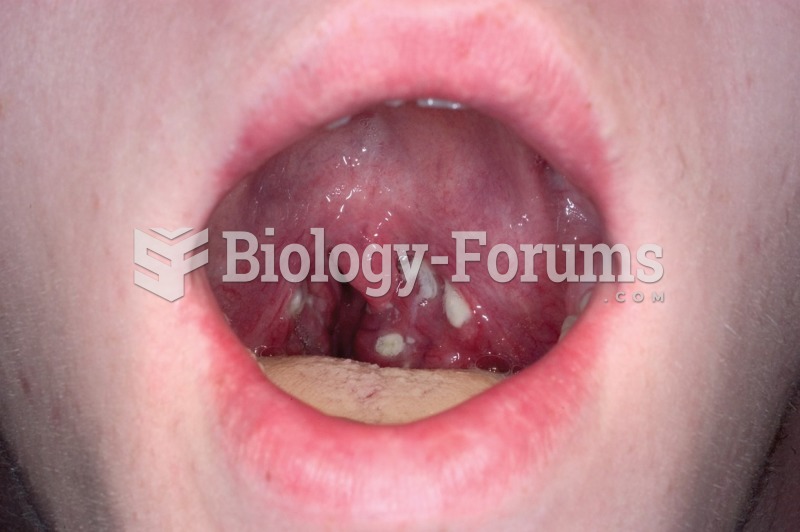 Inflammation of the oropharynx and petechiae (small red spots) on the soft palate caused by strep ...
Inflammation of the oropharynx and petechiae (small red spots) on the soft palate caused by strep ...